1. Background
Breast cancer is the most common cancer among women in Iran, and its epidemiological trends have changed significantly over the years. The age-standardized incidence rate (ASR) of breast cancer in Iran is approximately 21.33 cases per 100,000 individuals, with invasive ductal carcinoma being the most prevalent subtype (1). The median age at diagnosis ranges from 46 to 49 years, and a substantial proportion of cases (65.5% to 70.5%) are diagnosed at early stages (I and II) (2). Factors such as family history, the age at first childbirth, and reproductive history significantly influence the risk of developing breast cancer among Iranian women (3). Treatment options for cancer include surgery, chemotherapy, radiotherapy, and targeted therapies, which vary depending on the stage and type of cancer (4). Despite advancements in treatment and improvements in patient survival rates, there are significant disparities in access to critical care, particularly in low-and-middle-income countries (5). Recently, therapeutic strategies based on royal jelly (RJ) have garnered attention for their potential anticancer effects on tumor growth and their protective role against drug-induced toxicities (6). While the health benefits of RJ have been studied, its efficacy and safety in cancer treatment are not well established, and it is not adequately documented in clinical guidelines or specific research focused on breast cancer (7).
Royal jelly is a secretion produced by the glands of worker honey bees and serves as a special food for the queen bee. Its anti-inflammatory, antioxidant, and antimicrobial properties contribute to the queen’s significantly longer lifespan compared to worker bees. Several studies have indicated that RJ influences the production of various chemokines, antioxidants, and growth factors. Additionally, it affects the expression of cancer-related molecules in patients by inhibiting cell proliferation and inducing apoptosis in different types of malignant cells (6).
Cisplatin is a commonly used chemotherapeutic agent; however, it is associated with significant toxicity to the kidneys and liver. Research suggests that RJ may have protective effects against these side effects, potentially reducing cisplatin-induced kidney damage. In a study involving patients with cancer, those who received RJ exhibited lower levels of serum creatinine and urea compared to the control group, indicating its protective effect against renal toxicity (8).
When combined with cisplatin, RJ may enhance chemotherapy by improving parameters related to oxidative stress and apoptosis caused by cisplatin. This combination has been shown to increase the anti-apoptotic activity of liver cells and tubular epithelium. In animal studies, RJ has demonstrated the ability to reduce oxidative stress and apoptosis induced by cisplatin in both the kidneys and liver. It also enhances the activity of antioxidant enzymes and decreases lipid peroxidation, suggesting its potential as a protective agent during chemotherapy (9, 10). Furthermore, when RJ is combined with cisplatin, it has been found to improve blood parameters in rats. This indicates that RJ may help mitigate the adverse effects of cisplatin on blood health. Therefore, RJ could be considered a natural preventive food product to counteract cisplatin-induced changes in the blood during cancer treatment (11). Overall, the protective benefits of RJ against the side effects of cisplatin are promising, highlighting the need for further research in this area. This study specifically investigates the protective role of RJ in combination with cisplatin on the growth of the MCF-10A cell line at various concentrations and over different time periods.
2. Objectives
The present study mainly aimed to investigate the protective role of RJ against the lethal effects of cisplatin on normal breast tissue.
3. Methods
3.1. Preparation of Research Treatments
Cisplatin was sourced in 50 mL vials from Pars Daro Derman Trading Company, Iran, while RJ was obtained in 10 g packages from Moein Tajhiz Pajouh Company, Iran. To investigate the toxicity of the treatments, concentrations of 5 - 10 - 20 - 30 - 40 - 50 - 75 - 100 - 250 and 500 μg/mL were prepared from the original stock using a serial dilution method. Deionized distilled water served as the solvent for the treatments, with each concentration tested in triplicate.
3.2. MCF-10A Cell Line Culture and Counting
The MCF-10A cell line is an epithelial cell line that was isolated in 1984 from the mammary gland of a 36-year-old Caucasian woman with fibrocystic breasts (11). The MCF-10A breast cell line (ATCC code: CRL-10318) was purchased from the Pasteur Institute of Iran Cell Bank and cultured in DMEM/F12 medium (BioWest, France), supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) and 1% penicillin-streptomycin (Sigma-Aldrich, USA). The cells were maintained in an incubator at 37°C with 5% carbon dioxide. To count the mammary epithelial cells, the culture medium was first removed, and the flask containing the cells was washed with 1 mL of phosphate-buffered saline (PBS). The cells were then incubated with a 0.05% trypsin/EDTA solution for 5 minutes at 37°C. The culture medium was collected and centrifuged at 1200 RPM for 5 minutes. The resulting cell pellet was resuspended in 1 mL of culture medium. The cell count was determined using a 1:1 trypan blue/PBS solution on a counting slide (neubauer chamber) under a light microscope. After confirming the absence of contamination, only flasks with a confluency of over 90% were used for further studies.
3.3. Cell Treatment and Toxicity Assessment Using the MTT Assay Technique
To evaluate the toxicity of cisplatin and RJ on cells, 10,000 cells were cultured in each well of a 96-well plate. The cells were treated with different concentrations of a specific compound (designated as 5 - 10 - 20 - 30 - 40 - 50 - 75 - 100 - 250), in addition to 500 μg/mL of cisplatin and RJ, both individually and in combination. This treatment occurred for 24, 48, and 72 hours at 37°C in a 5% carbon dioxide atmosphere. The MTT assay technique was employed to assess the toxicity of these treatments on the cells. This technique is based on the decomposition of yellow tetrazolium salts in the mitochondria of living cells, converting them into formazan. This conversion allows for the detection of viable cells. Insoluble formazan compounds turn purple in the presence of EDTA and can be quantified by measuring optical absorption at a wavelength of 570 nm. To perform the MTT assay, 10 μL of MTT solution (5 mg/mL, Sigma) was added to the cell supernatant, and the plates were incubated for 3 hours at 37°C. After incubation, the cell supernatant was carefully removed, and 100 μL of DMSO (Sigma) was added to each well. The absorbance was then measured using an ELISA Reader (Biotech, Germany).
The percentage of cell survival was calculated using the following equation: (Mean absorbance of treated sample)/(mean absorbance of negative control sample) × 100 = Percentage of cell survival
The toxicity of the treatments was determined using this equation: Percentage of cell survival - 100 = Percentage of cytotoxicity
3.4. Statistical Analysis
Statistical analysis of the data was conducted using Graphpad prism 9 software. One-way ANOVA was employed along with post-hoc tests, including variance evaluation using LSD and Tukey tests. A P-value ≤ 0.05 was considered statistically significant
4. Results
4.1. Examination of Treatment Growth Changes Compared to the Control Group
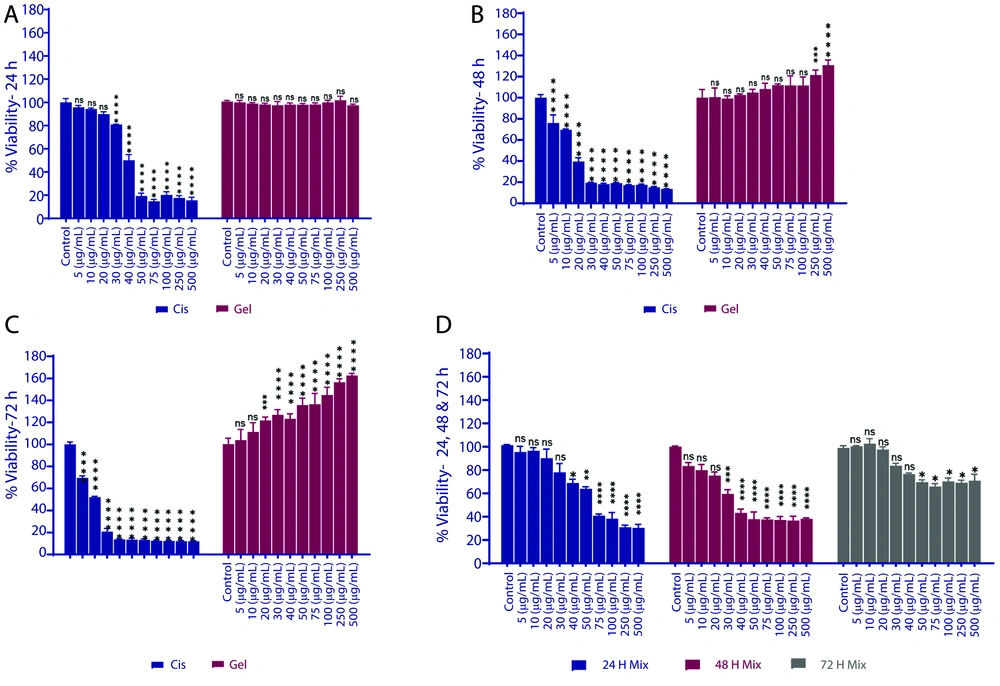
The differences in growth rates at various concentrations were statistically evaluated in comparison to the control group. To investigate the impact of cisplatin toxicity and the protective effect of RJ on the survival and growth of normal breast epithelial cells, we assessed the viability of MCF-10 cells at different concentrations of cisplatin. The results indicated that cell viability decreased after 24 hours of exposure to concentrations greater than 30 μg/mL (P = 0.0008). Additionally, after 48 and 72 hours, cell viability declined at concentrations exceeding 5 μg/mL when compared to the control group (P < 0.0001). The average percentage of cell viability with cisplatin is presented in Table 1.
| Dose Cis Platin (µg/mL) | 24 h (% Viability) | 48 h (% Viability) | 72 h (% Viability) | |||
|---|---|---|---|---|---|---|
| Mean ± SD | P-Value | Mean ± SD | P-Value | Mean ± SD | P-Value | |
| 0 | 100.000 ± 3.171 | - | 100 ± 2.942 | - | 100.000 ± 2.207 | - |
| 5 | 95.086 ± 3.416 | NS | 76.029 ± 7.813 | < 0.0001 | 69.699 ± 1.830 | < 0.0001 |
| 10 | 94.885 ± 1.189 | NS | 69.662 ± 0.699 | < 0.0001 | 52.082 ± 0.844 | < 0.0001 |
| 20 | 89.093 ± 1.624 | NS | 39.6463.574 ± | < 0.0001 | 20.877 ± 2.890 | < 0.0001 |
| 30 | 80.548 ± 1.346 | 0.0008 | 19.529 ± 0.403 | < 0.0001 | 13.753 ± 0.502 | < 0.0001 |
| 40 | 50.040 ± 4.908 | < 0.0001 | 18.138 ± 0.834 | < 0.0001 | 13.534 ± 1.245 | < 0.0001 |
| 50 | 19.268 ± 2.444 | < 0.0001 | 19.047 ± 0.723 | < 0.0001 | 13.260 ± 0.905 | < 0.0001 |
| 75 | 14.722 ± 1.583 | < 0.0001 | 17.1210.668 ± | < 0.0001 | 12.438 ± 0.741 | < 0.0001 |
| 100 | 20.233 ± 2.672 | < 0.0001 | 17.495 ± 0.642 | < 0.0001 | 12.2740.528 ± | < 0.0001 |
| 250 | 17.578 ± 2.053 | < 0.0001 | 15.141 ± 0.668 | < 0.0001 | 12.110 ± 0.577 | < 0.0001 |
| 500 | 15.447 ± 2.760 | < 0.0001 | 13.590 ± 0.0926 | < 0.0001 | 11.945 ± 0.528 | < 0.0001 |
Abbreviation: NS, not significant.
The growth of cells cultured in a medium containing RJ showed little change compared to the control group during the first 24 hours (P > 0.05). However, after 48 hours of exposure, an increase in growth was observed at RJ concentrations of 250 μg/mL (P = 0.0002) or higher. After 72 hours, a growth increase was noted at concentrations starting from 20 μg/mL (P = 0.0002). The average percentage of cell growth with RJ is presented in Table 2.
| Dose RJ (µg/mL) | 24 h (% Viability) | 48 h (% Viability) | 72 h (% Viability) | |||
|---|---|---|---|---|---|---|
| Mean ± SD | P-Value | Mean ± SD | P-Value | Mean ± SD | P-Value | |
| 0 | 100 ± 0.426 | - | 100 ± 7.803 | - | 100 ± 5.507 | - |
| 5 | 99.261 ± 3.995 | NS | 100.534 ± 8.609 | NS | 103.926 ± 9.645 | NS |
| 10 | 98.211 ± 4.416 | NS | 99.889 ± 2.290 | NS | 111.295 ± 8.424 | NS |
| 20 | 98.797 ± 7.046 | NS | 102.572 ± 2.223 | NS | 121.763 ± 3.067 | 0.0002 |
| 30 | 97.722 ± 5.837 | NS | 104.914 ± 2.978 | NS | 126.854 ± 8.281 | < 0.0001 |
| 40 | 98.336 ± 5.551 | NS | 108.066 ± 5.664 | NS | 129.309 ± 8.854 | < 0.0001 |
| 50 | 97.637 ± 3.498 | NS | 111.858 ± 1.251 | NS | 135.675 ± 6.389 | < 0.0001 |
| 75 | 98.437 ± 3.269 | NS | 111.538 ± 9.182 | NS | 136.433 ± 9.935 | < 0.0001 |
| 100 | 99.548 ± 5.014 | NS | 111.538 ± 8.235 | NS | 144.835 ± 7.148 | < 0.0001 |
| 250 | 101.110 ± 4.721 | NS | 121.475 ± 4.735 | 0.0002 | 156.405 ± 3.247 | < 0.0001 |
| 500 | 97.273 ± 4.978 | NS | 130.769 ± 4.975 | < 0.0001 | 162.603 ± 1.992 | < 0.0001 |
Abbreviations: RJ, royal jell; NS, not significant.
Using a 1:1 combination of RJ and cisplatin, we observed a significant difference in cell growth compared to the control sample during the first 24 hours at a concentration of 40 μg/mL (P = 0.0199). This effect continued after 48 hours at a concentration of 30 μg/mL (P = 0.0003) and after 72 hours at concentrations of 50 μg/mL (P = 0.0236) and higher. Interestingly, after 72 hours, the decrease in growth was less pronounced and remained stable at higher concentrations (Table 3).
| Dose MIX (µg/mL) | 24 h (% Viability) | 48 h (% Viability) | 72 h (% Viability) | |||
|---|---|---|---|---|---|---|
| Mean ± SD | P-Value | Mean ± SD | P-Value | Mean ± SD | P-Value | |
| 0 | 99.99 ± 7.656 | - | 100 ± 8.030 | - | 100 ± 13.479 | - |
| 5 | 97.794 ± 16.154 | NS | 90.1290 ± 8.278 | NS | 102.099 ± 20.991 | NS |
| 10 | 98.516 ± 9.120 | NS | 84.322 ± 11.844 | NS | 104.963 ± 20.565 | NS |
| 20 | 90.107 ± 15.201 | NS | 75.225 ± 9.1467 | NS | 102.671 ± 20.179 | NS |
| 30 | 75.359 ± 8.139 | NS | 60.258 ± 11.340 | 0.0003 | 83.778 ± 10.310 | NS |
| 40 | 69.258 ± 3.741 | 0.0199 | 43.225 ± 3.292 | < 0.0001 | 73.854 ± 2.064 | NS |
| 50 | 63.248 ± 10.181 | 0.0012 | 37.806 ± 6.293 | < 0.0001 | 69.656 ± 2.0106 | 0.0236 |
| 75 | 40.827 ± 1.485 | < 0.0001 | 37.677 ± 1.244 | < 0.0001 | 69.839 ± 2.623 | 0.0256 |
| 100 | 33.219 ± 5.451 | < 0.0001 | 37.161 ± 3.0233 | < 0.0001 | 70.289 ± 2.882 | 0.0301 |
| 250 | 30.935 ± 1.796 | < 0.0001 | 36.645 ± 3.658 | < 0.0001 | 69.274 ± 2.064 | 0.0201 |
| 500 | 30.305 ± 3.150 | < 0.0001 | 38.193 ± 0.805 | < 0.0001 | 70.658 ± 5.461 | 0.0411 |
Abbreviation: NS, not significant.
Figure 1 shows a graphical comparison of cell growth over time in single and combined treatments.
Cell viability of MCF-10A cells treated with cisplatin, royal jelly (RJ), and their combination over time. A, Cell viability after 24 hours of treatment with cisplatin, RJ, or their combination; B, cell viability after 48 hours of treatment at various concentrations; C, cell viability after 72 hours of exposure to the treatments; D, cell viability for the 1:1 combination of cisplatin and RJ at different time points. X-axis: Concentration (μg/mL). Y-axis: Percentage of cell viability (%). Significance is indicated by asterisks: * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001 compared to the control group.
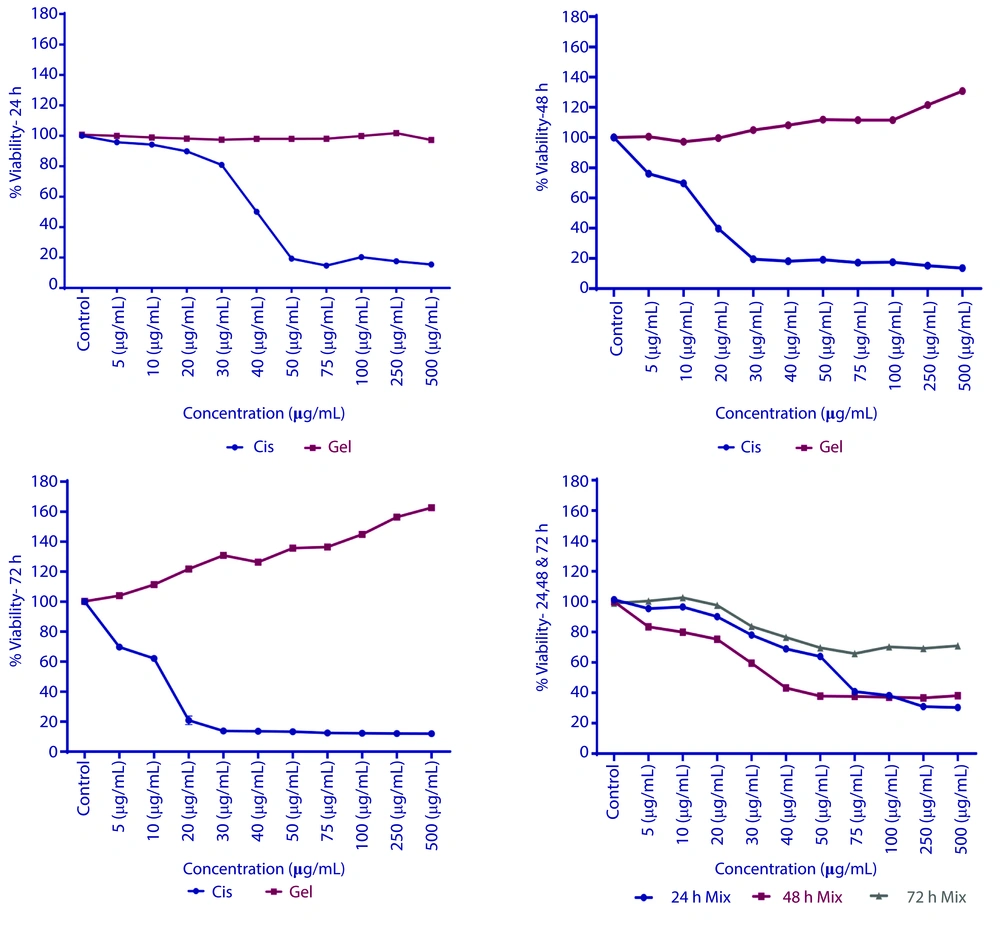
Statistical calculations indicated that a 50% mortality rate was observed in cells treated with cisplatin at concentrations of 40.11 μg/mL after 24 hours; 14.4 μg/mL after 48 hours, and 10.43 μg/mL after 72 hours. In contrast, treatment with RJ showed no significant change in cell growth after 24 hours. However, significant growth was noted after 48 hours at concentrations greater than 100 μg/mL. By 72 hours, the half-maximal effective concentration (EC50) was determined to be 246.25 μg/mL. When combining both treatments, the half-maximal inhibitory concentration (IC50) was 68.82 μg/mL after 24 hours and 37.61 μg/mL after 48 hours. However, after 72 hours, more than 70% of the cells exhibited consistent growth. The results of these treatments are illustrated in Figure 2.
The effects of cisplatin (decreased viability), royal jelly (RJ) (increased viability at higher concentrations), and their combination (initial decrease followed by stabilization). Additionally, ensured the figure includes distinct data points for each treatment (cisplatin, RJ, combination) across time points (24, 48, 72 hours), with error bars for standard deviation.
5. Discussion
Royal jelly has demonstrated protective effects against the toxicity induced by cisplatin through various mechanisms. This natural compound is rich in antioxidants, which help neutralize the free radicals generated by cisplatin, thereby preventing cellular damage (12). Studies have indicated that the biochemical, hematological, and histological changes caused by cisplatin were alleviated in mice treated with RJ, suggesting its potential as a preventive agent against cisplatin-induced hepatotoxicity (13).
Furthermore, RJ helps mitigate the inflammatory responses induced by cisplatin, resulting in reduced levels of inflammatory markers and improved tissue integrity (6). It also restores the activity of important enzymes, such as paraoxonase-1 and aryl esterase, which are diminished by cisplatin, thereby enhancing liver function and reducing toxicity (14). Additionally, RJ promotes cell survival and inhibits apoptosis in damaged cells. This effect is associated with a significant increase in the mRNA levels of the transcription factor E2f1, which aids in maintaining cellular integrity during chemotherapy (15).
In the present study, RJ demonstrated significant cell growth after 48 hours at concentrations exceeding 100 μg/mL, with growth continuing to increase after 72 hours. This effect is likely attributed to the bioactive components found in Orsolic and Jazvinscak Jembrek reported that 10-hydroxy-2-decenoic acid (10-HDA) and major RJ proteins (MRJPs) possess antioxidant, anti-inflammatory, and estrogenic properties. These characteristics may contribute to RJ’s effectiveness in promoting breast health and potentially inhibiting the proliferation of breast cancer cells (16).
When a combination of cisplatin and RJ was administered, the cells initially exhibited a decrease in growth, followed by consistent growth at all three observed time points. These changes are likely influenced by RJ’s role in hormonal regulation and its impact on breast tissue structure. A study by Liu et al. found that RJ affects two key hormones in breast tissue: Estrogen and progesterone. In their research conducted on mice, they observed that RJ treatment improved the structure of mammary gland tissue and regulated serum levels of these hormones. Additionally, RJ positively impacts the morphology of breast tissue, enhancing the expansion of acinar and ductal structures at certain doses. Therefore, it can be concluded that RJ has potential benefits in maintaining breast health (17).
The protective properties of RJ are attributed to its various bioactive compounds, including proteins, vitamins, and polyphenols. These components are known to promote cell health, enhance antioxidant activity, and protect tissues, contributing to the maintenance of normal breast tissue function (16).
While RJ is safe for most individuals, it can lead to some potential side effects. These may include allergic reactions, which can manifest as skin rashes, asthma, or even anaphylaxis. Additionally, gastrointestinal issues, such as diarrhea, may occur (18). Royal jelly may also exhibit estrogen-like hormonal effects, which could result in hormonal imbalances (19). Furthermore, there is a risk of drug interactions, particularly for individuals who are diabetic or sensitive to hormones (20).
5.1. Conclusions
In summary, RJ appears to be more effective than other natural compounds. While various supplements offer general health benefits, the unique composition of RJ — rich in essential nutrients and its ability to promote hormonal balance — provides a more focused approach to maintaining breast health. The combined use of cisplatin and RJ has shown protective effects on normal breast cells, helping to preserve healthy breast tissue. Furthermore, additional molecular studies are necessary to explore intracellular changes and to gain a deeper understanding of RJs role in supporting patients undergoing chemotherapy.