1. Background
In 2023, the FDA approved cisplatin for the 45th time, underscoring its role as a cornerstone in cancer therapy. Widely used to treat early-stage ovarian cancer, non-small cell lung cancer (NSCLC), head and neck cancer, advanced bladder cancer, and testicular cancer, cisplatin achieves a 100% cure rate when cancer is detected early. Although its precise mechanism remains incompletely understood, cisplatin induces cancer cell death through four key processes: Cellular uptake, activation by aquation, DNA binding, and processing of DNA lesions. Notably, cisplatin causes significant mitochondrial DNA (MT-ND1) damage, triggering mitochondria-mediated apoptosis (1). Cisplatin binds to DNA, causing damage, and induces mitochondria-mediated apoptosis. The MT-ND1 is greatly impacted by cisplatin (2). Cisplatin-sensitive cells contain higher mitochondrial content and elevated levels of mitochondrial reactive oxygen species (mtROS) compared to cisplatin-resistant cells. Increasing mtROS by inhibiting or destroying the ROS-protective uncoupling protein UCP2 enhances cisplatin-induced apoptosis (3).
To mitigate cisplatin’s side effects, recent studies have proposed royal jelly (RJ) as an effective antioxidant capable of inhibiting free radicals. The RJ, produced and secreted by the hypopharynx and mandibular glands of worker honey bees (Apis mellifera), contains essential compounds such as free amino acids, proteins, sugars, fatty acids, minerals, and vitamins. These confer multiple biological activities, including vasodilation, blood pressure reduction, cholesterol lowering, antimicrobial, anti-allergenic, anti-inflammatory, and antioxidant properties (4).
Chromobox protein 3 homolog/heterochromatin protein γ (CBX3/HP1γ), a member of the evolutionarily conserved heterochromatin protein family, is a DNA-binding factor that regulates gene transcription. Overexpression of this gene promotes cancer cell proliferation and plays a central role in tumorigenesis. The CBX3 expression is a poor prognosis marker in human cancers, including glioblastoma multiforme (GBM), NSCLC, ovarian cancer, breast cancer, osteosarcoma, hepatocellular carcinoma, gastric cancer, pancreatic adenocarcinoma, and prostate cancer (5).
NADH dehydrogenase subunit 1, encoded by MT-ND1, is the largest subunit of respiratory complex I (6). Located in the inner mitochondrial membrane, it facilitates electron transfer and proton pumping. Variations in this gene are associated with diseases such as Alzheimer’s disease, colorectal cancer, Leber optic atrophy, Leigh syndrome, mitochondrial encephalopathy, lactic acidosis, stroke-like episodes, diabetes, dystonia, and hypertrophic cardiomyopathy (7).
2. Objectives
This study aimed at investigating the effects of cisplatin, RJ, and their combination on the growth, proliferation, and apoptosis of A549 lung cancer cells, focusing on CBX3 and MT-ND1 gene expression.
3. Methods
3.1. Preparation of Cisplatin and Royal Jelly
Cisplatin, obtained in a 50 ml vial from Pars Daro Derman Trading Company, and RJ, supplied in a 10 g package from Moein Tajhiz Pajoh Company, were used. Treatment solutions were prepared at concentrations of 5, 10, 20, 30, 40, 50, 75, 100, 250, and 500 μg/mL for both cisplatin and RJ. Cisplatin was dissolved in deionized distilled water to ensure biocompatibility with the A549 cell line and to avoid solvent-related cytotoxicity or interference with gene expression, which can occur with DMSO or DMF at certain concentrations. Solutions were freshly prepared to maintain stability and efficacy.
3.2. Cell Cultivation and Treatment
The adherent human lung carcinoma cell line A549 (ATCC CRM-CCL-185) was propagated in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and maintained at 37°C with 95% relative humidity and 5% CO2. For the MTT assay, 104 cells were seeded into each well of a 96-well plate and allowed to adhere for 24 hours. Cells were treated with cisplatin, RJ, or a 1:1 combination at concentrations of 5, 10, 20, 30, 40, 50, 75, 100, 250, and 500 μg/mL for 24, 48, or 72 hours. After treatment, the medium was replaced with 100 μL of fresh DMEM containing 10 μL of 5 mg/mL MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] solution (Sigma-Aldrich, USA). Plates were incubated for 4 hours at 37°C, allowing viable cells to reduce MTT to formazan crystals. The medium was removed, and 100 μL of DMSO was added to dissolve the formazan. Absorbance was measured at 570 nm using a microplate reader (BioTek, USA). Cell viability was calculated as a percentage relative to untreated controls, with three replicates per condition.
3.3. Cell Treatment and Gene Expression Examination
A549 cells (1 × 106 cells/well) were seeded in 6-well plates for 24 hours, followed by treatment with IC50 and EC50 concentrations for 48 hours. Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. To eliminate contamination of genomic DNA, RNA samples were treated with DNase I (Fermentas, Burlington, Canada). RNA quantity and integrity were assessed using a NanoDrop spectrophotometer (Thermo Scientific, USA) and agarose gel electrophoresis, ensuring an A260/A280 ratio of 1.8 - 2.0. cDNA was synthesized from 1000 ng of RNA using the RevertAid™ First Strand cDNA Synthesis Kit (Fermentas, Burlington, Canada). Expression levels of B2M (housekeeping gene), CBX3, and MT-ND1 were quantified using SYBR Green-based qPCR in a 25 μL reaction containing 12.5 μL of master mix (Bioneer, Korea), 1 μL of cDNA, 1 μL of forward and reverse primers (10 μM, Table 1), and DEPC-treated water. Reactions were performed in triplicate on a Rotor-Gene system with the following cycling conditions: 95°C for 10 minutes, followed by 40 cycles of 95°C for 20 seconds, 57°C for 40 seconds, and 72°C for 40 seconds. No-template and minus-reverse-transcriptase controls were included to confirm the absence of DNA contamination.
| Symbol | 5' Sequence 3' |
|---|---|
| CBX3 | |
| Forward primer | GAGATGCTGCTGACAAACCA |
| Reverse primer | TATTTGCCTCTTTCGCCAGC |
| MT-ND1 | |
| Forward primer | CCACCTCTAGCCTAGCCGTTTAC |
| Reverse primer | GGGTCATGATGGCAGGAGTAAT |
| β-2 microglobulin | |
| Forward primer | CCACTGAAAAAGATGAGTATGCCT |
| Reverse primer | CCAATCCAAATGCGGCATCTTCA |
Primer Sequence Used in the Study
Primers for CBX3 and B2M (Table 1) were designed using Primer3 software and validated for specificity using NCBI BLAST against the human RefSeq mRNA database. Primers specific to MT-ND1 (Table 1) were obtained from previously published studies (8, 9).
3.4. Detection of Apoptosis Using Annexin V-FITC Test
A549 cells (1 × 105 cells/well) were seeded in 6-well plates and cultured with IC50 and EC50 concentrations for 48 hours. Untreated controls were included. After the treatment, cells were washed twice with PBS. Apoptosis and necrosis were assessed using an Annexin V-FITC kit (Roch, Penzberg, Germany). The instructions were followed carefully. Flow cytometry from Biocompare, San Francisco, and USA was used for analysis and data were processed using Flow Jo software.
3.5. Statistical Analysis
Data were analyzed using GraphPad Prism 9 software and one-way ANOVA. Gene expression levels in treated and untreated cells were compared with Tukey’s HSD post-hoc test used for pairwise comparisons. Results were reported as mean ± standard deviation (SD) from three replicates. A P-value < 0.05 was considered statistically significant.
4. Results
4.1. Cell Viability
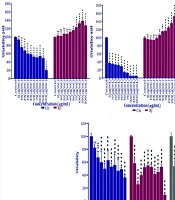
The cytotoxic and anti-proliferative effects of cisplatin and RJ are shown in Figure 1. Cisplatin's effects on cell growth were studied after 24, 48, and 72 hours, as were those of RJ. Cisplatin's concentration and treatment duration influenced cell growth inhibition. After 24 hours with a concentration of 10 µg/mL, and 48 and 72 hours with a minimum of 5 µg/mL, cell viability significantly decreased compared to the control group.
Cell viability of A549 cells after treatment with cisplatin, royal jelly (RJ), or a 1:1 combination at 24, 48, and 72 hours. The X-axis represents treatment concentrations (μg/mL), and the Y-axis shows cell viability (% of control). Statistical significance compared to the control group is indicated by * P < 0.05, ** P < 0.01, or *** P < 0.001.
IC50 values for cisplatin were 72.89, 10.06, and 1.68 μg/mL at 24, 48, and 72 hours, respectively. The RJ increased cell proliferation at 50, 40, and 30 µg/mL, with significant growth compared to controls. EC50 values for RJ were 557.23, 489.03, and 173.92 µg/mL at 24, 48, and 72 hours, respectively. The 1:1 combination treatment significantly reduced cell viability, with IC50 values of 66.75 μg/mL at 24 hours, 30.83 μg/mL at 48 hours, and 6.85 μg/mL at 72 hours.
4.2. Expression of Chromobox Protein 3 Homolog and Mitochondrial DNA Genes After 48 Hours
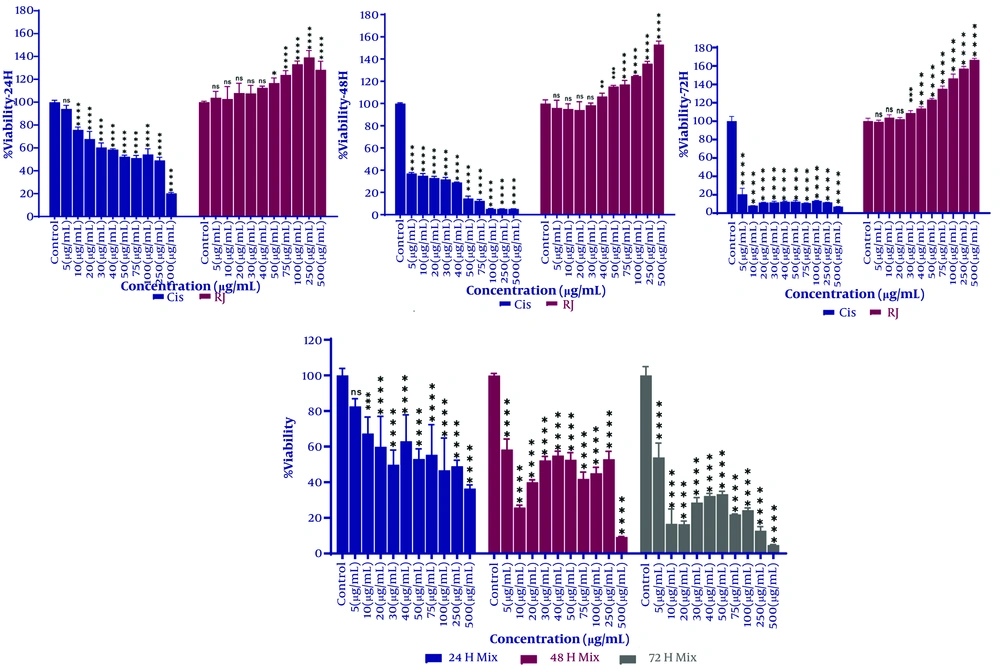
Cells were treated with 10 μg/mL cisplatin (IC50), 489 μg/mL RJ (EC50), or a 1:1 combination for 48 hours. RNA was extracted, cDNA synthesized, and gene expression analyzed via qPCR. Cisplatin significantly reduced CBX3 and MT-ND1 expression (P < 0.05), while RJ increased expression compared to controls (P < 0.05), consistent with MTT assay results. The combination treatment increased gene expression compared to cisplatin alone but decreased it compared to RJ (P < 0.05). Results are shown in Figure 2.
Expression of chromobox protein 3 homolog (CBX3) and mitochondrial DNA (MT-ND1) genes in A549 cells treated with cisplatin, royal jelly (RJ), or a 1:1 combination for 48 hours. The X-axis represents treatment conditions, and the Y-axis shows relative gene expression (fold change). Cisplatin decreased CBX3 (P = 0.034) and MT-ND1 (P = 0.006) expression, while RJ increased CBX3 (P = 0.009) and MT-ND1 (P < 0.0001). The combination increased expression compared to cisplatin (CBX3: P = 0.046; MT-ND1: P = 0.0007) but decreased it compared to RJ. Significance is indicated by * P < 0.05, ** P < 0.01, or *** P < 0.001, **** P < 0.0001.
4.3. Apoptosis Induction
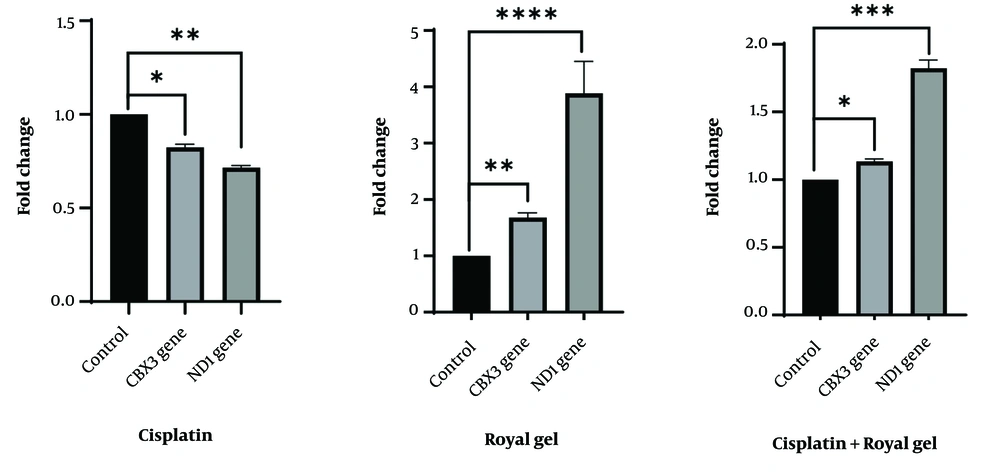
Apoptosis in A549 cells was assessed after 48 hours of treatment with cisplatin, RJ, or their 1:1 combination at IC50 and EC50 concentrations. Flow cytometry showed 79.7% healthy cells in the control group, compared to 18.3% for cisplatin, 73.4% for RJ, and 25.5% for the combination. Statistically significant differences were observed for cisplatin (P = 0.0008), RJ (P = 0.0247), and the combination (P = 0.0016) compared to controls. Gene expression patterns and flow cytometry results were consistent, confirming the reliability of the treatments’ effects (Figure 3).
Flow cytometry analysis of A549 cells treated with cisplatin, royal jelly (RJ), or a 1:1 combination for 48 hours. The X-axis represents treatment conditions (control, cisplatin, RJ, combination), and the Y-axis shows the percentage of cells in each state (healthy, apoptotic, necrotic). The apoptosis rate was 54.2% for cisplatin, 11.9% for RJ, and 53.5% for the combination, with necrosis rates of 25.8%, 13%, and 14.4%, respectively; * P < 0.05, ** P < 0.01, or *** P < 0.001.
5. Discussion
Cancer, a leading cause of death worldwide, is commonly treated with radiotherapy, surgery, and chemotherapy, all of which can cause side effects. A healthy lifestyle, particularly a proper diet, is crucial for cancer prevention. (10). Combining plant-derived compounds with chemotherapy can enhance efficacy while reducing toxicity to healthy tissues. The RJ, with its potent antioxidant properties and ability to inhibit free radicals, has garnered significant attention (2). Despite ongoing research into natural products combined with anti-cancer drugs, definitive results remain elusive.
The RJ contains numerous bioactive compounds and exhibits various biological and pharmacological properties. Due to its low toxicity compared to other bee products (10), this study compared its effects with cisplatin on A549 cell viability, CBX3 and MT-ND1 gene expression, and apoptosis/necrosis induction
Cisplatin alone significantly reduced cell growth, while RJ increased proliferation at specific concentrations (50 μg/mL at 24 hours, 40 μg/mL at 48 hours, and 30 μg/mL at 72 hours) with statistically significant differences compared to controls. In a study by Salazar-Olivo and Paz-Gonzalez, extracted crude protein from RJ increased the population doubling rate of Tn-5B1-4 insect cells by 6.5 times per milligram of protein, compared to 2.55 times for fetal bovine serum, suggesting that RJ’s bioactive compounds promote cell growth, differentiation, and survival in various cell types (11).
The compound 10-hydroxy-2-decenoic acid, structurally similar to mammalian estrogen, may contribute to RJ’s proliferative effects and reduce side effects of cancer treatment (12). This fatty acid exhibits anti-cancer, anti-inflammatory, immune-modulating, and antimicrobial properties (13). Although some studies have suggested estrogenic activity (14-16), Ishida et al. found that RJ does not activate ERα or ERβ, indicating that its estrogen-like effects are not mediated through these receptors (17).
The 1:1 combination of cisplatin and RJ significantly reduced cell growth, mirroring cisplatin’s effects, indicating RJ’s cytotoxic potential. Similar results were observed in bladder cancer cells, where RJ reduced viability, migration, and matrix metalloproteinase 9 (MMP-9) expression and protein levels in HTB 5637 cells (18).
Overexpression of CBX3 is linked to cancer progression (19, 20). Niu et al. found correlations between CBX3 expression and survival prognosis, DNA methylation, protein phosphorylation, and immune cell infiltration, suggesting CBX3 as a biomarker and therapeutic target (19). MT-ND1, encoding the largest subunit of mitochondrial complex I, is critical for oxidative phosphorylation. Its mutations make it a significant cancer biomarker (21).
Flow cytometry showed apoptosis rates of 1.17% for cisplatin, 1.66% for RJ, and 6.61% for the combination, with 53.5% of combination-treated cells in late apoptosis and 14.4% in necrosis. The combination reduced viable cells to 25.5%, compared to 18.3% for the cisplatin treatment alone.
In line with the present study, Icariside II (IS) combined with cisplatin inhibits cell proliferation and induces apoptosis in NSCLC without significant toxicity. The IS enhances cisplatin-induced apoptosis partly by promoting endoplasmic reticulum (ER) stress signaling (22).
The RJ proteins, including RJG-1 (a glucosylceramidase) and RJG-2 (major RJ protein 1), play key roles in cell death; RJG-1 disrupts cell membrane glucosylceramide, causing necrosis (23). Additionally, RJ induces programmed cell death in acute lymphoblastic leukemia (ALL) and nervous system cancers (24). In neuroblastoma and glioblastoma cells, increased apoptosis is linked to changes in macromolecular composition, protein structure, and phosphorylation (25).
This study was limited to RJ’s effects on cancer cell viability, gene expression, and apoptosis. Further research is needed on the antioxidant and anti-inflammatory roles of its combination with cisplatin and the molecular mechanisms behind the increased apoptosis and related immune responses.
5.1. Conclusions
Cisplatin’s side effects, including excessive free radical production, can damage healthy tissues. The RJ, a potent free radical scavenger, may mitigate these effects. This study demonstrates that combining cisplatin with RJ reduces A549 cell viability comparably to cisplatin alone while inducing a sixfold increase in apoptosis. These findings suggest that RJ could enhance cisplatin’s efficacy in lung cancer treatment. Further clinical studies are warranted to explore the therapeutic potential of this combination and its impact on immune responses.