1. Background
Lung cancer is the second most common diagnosed cancer and the leading cause of cancer-related deaths in the world. In Indonesia, lung cancer is the leading cause of cancer-related deaths, estimated at 30,000 cases (13.2%). The incidence rate for lung cancer in males is 20.1 per 100,000 population, with 25,943 new cases and an average death rate of 11.4 per 100,000 population (1). Epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) such as first generation (gefitinib, erlotinib), and second generation (afatinib), or third generation (osimertinib) are available for advanced-stage non-squamous non-small cell lung cancer (NSCLC) harboring positive EGFR mutation, for common mutation or uncommon mutation (2). Since 2018, afatinib was approved for the universal health coverage (UHC) in Indonesia, however, the efficacy and safety have not been elaborated.
2. Objectives
The aim of the study was to evaluate real world data of efficacy and safety of afatinib as the first line treatment of EGFR mutation positive NSCLC, and to seek the efficacy data regarding dose adjustment of afatinib, under the universal health coverage program in Indonesia.
3. Methods
3.1. Study Design and Subjects
This retrospective observational cohort analysis was based on the medical records from the Thoracic Oncology Outpatient clinic in Persahabatan Hospital, a tertiary respiratory referral hospital, between January 2018 and December 2021. A total of 143 participants were recruited using total sampling technique to achieve a minimum required sample size of 77 as a minimum sample.
The inclusion criteria were all patients who have been diagnosed cytologically and/or histopathologically with lung adenocarcinoma with common EGFR mutations in exon 19 and exon 21 L858R or dual mutations and who received EGFR-TKI afatinib as the first line between January 1, 2018, and December 31, 2021, under the Indonesian UHC program. Afatinib was approved for locally advanced or metastatic NSCLC with EGFR exon 19 deletion, exon 21 (L858R and L861Q), 18 (G719X), or 20 (S768I) substitution mutation in patients who have not received prior TKI therapy (3).
Exclusion criteria included loss of contact, afatinib as a second or later line, receiving afatinib less than three months, lung cancer as a metastasis from another organ, and incomplete data. These selection processes are some efforts prior to study conduction to address and minimize potential bias.
3.2. Data Collection
Epidermal growth factor receptor mutation results were obtained from a certified laboratory received from the database of patients. Method of testing was real-time polymerase chain reaction (RT-PCR) and next-generation sequencing (NGS), either from cytological or histopathological samples. All participants in this study initially received 40 mg dose of afatinib and then some had dose reduced to 20 or 30 mg. The standard follow-up time and image modalities using brain or thorax computed tomography (CT)-scan every three months.
3.3. Statistical Analysis
Data was entered into a master table using the statistical package for the social sciences (SPSS) version 25. The collected data included clinical characteristics, disease progression, toxicity, history of dose reduction, and survival analysis [progression-free survival (PFS), overall survival (OS), and one-year survival rate]. The data were summarized and analyzed as mean ± standard deviation (SD) for normally distributed variables or median with interquartile range for non-normally distributed data. Survival analysis was conducted using Kaplan-Meier and testing statistical significance for each independent variable using the Log-Rank test. The results were presented at 95% confidence interval (CI).
4. Results
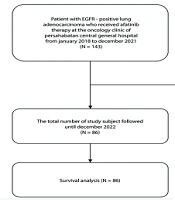
According to the data collection process, 143 patients with EGFR-mutated lung adenocarcinoma who had received afatinib between January 2018 and December 2021 were initially identified. However, 57 participants were excluded due to receiving afatinib not as a first-line treatment (12 participants), incomplete medical records (15 participants), very poor performance status (20 participants), and loss of contact (10 participants). Ultimately, a total of 86 participants were included for the study. Participant’s recruitment flow is illustrated in Figure 1.
4.1. Characteristics of the Patients
The baseline characteristic of the participants are presented in Table 1. Approximately 78% of the total study participants were diagnosed stage IVA at the initiation of afatinib therapy. Among all EGFR mutations, exon 19 deletion were found in more than 50% of participants. Pleural metastasis accounting for nearly 60% of the total participants. The average follow-up time was 16 months (range 3 - 42 months).
| Baseline Characteristics | No. (%) |
|---|---|
| Age (y) | |
| ≥ 65 | 16 (18.6) |
| < 65 | 70 (81.4) |
| Gender | |
| Male | 42 (48.8) |
| Female | 44 (51.2) |
| Ethnic | |
| Javanese | 70 (81.4) |
| Non-Javanese | 16 (18.6) |
| Risk factors | |
| Family history of malignancy | 15 (17.4) |
| Smoking history | 41 (47.7) |
| Brinkmann index | |
| Light | 12 (29.3) |
| Moderate | 15 (36.6) |
| Heavy | 14 (34.1) |
| Stage | |
| IIIA - IIIB | 10 (11.6) |
| IVA | 67 (77.9) |
| IVB | 9 (10.5) |
| Performance status | |
| 0 - 1 | 69 (80.2) |
| 2 - 4 | 17 (19.8) |
| EGFR mutation | |
| Exon 19 | 52 (60.5) |
| Exon 21 L858R | 25 (29.0) |
| Exon 21 L861Q | 3 (3.5) |
| Combination | 6 (7.0) |
| Site of metastasis | |
| Pleura | 51 (59.3) |
| Pericard | 6 (7.0) |
| Contralateral lung | 16 (18.6) |
| Brain | 17 (19.8) |
| Bone | 18 (20.9) |
| Liver | 7 (8.1) |
| Dose reduction (mg) | 37 (43.0) |
| 30 | 6 (16.2) |
| 20 | 31 (83.8) |
Participants’ Characteristics (N = 86)
4.2. Analysis of Progression-Free Survival, OS, and One-Year Survival Rate
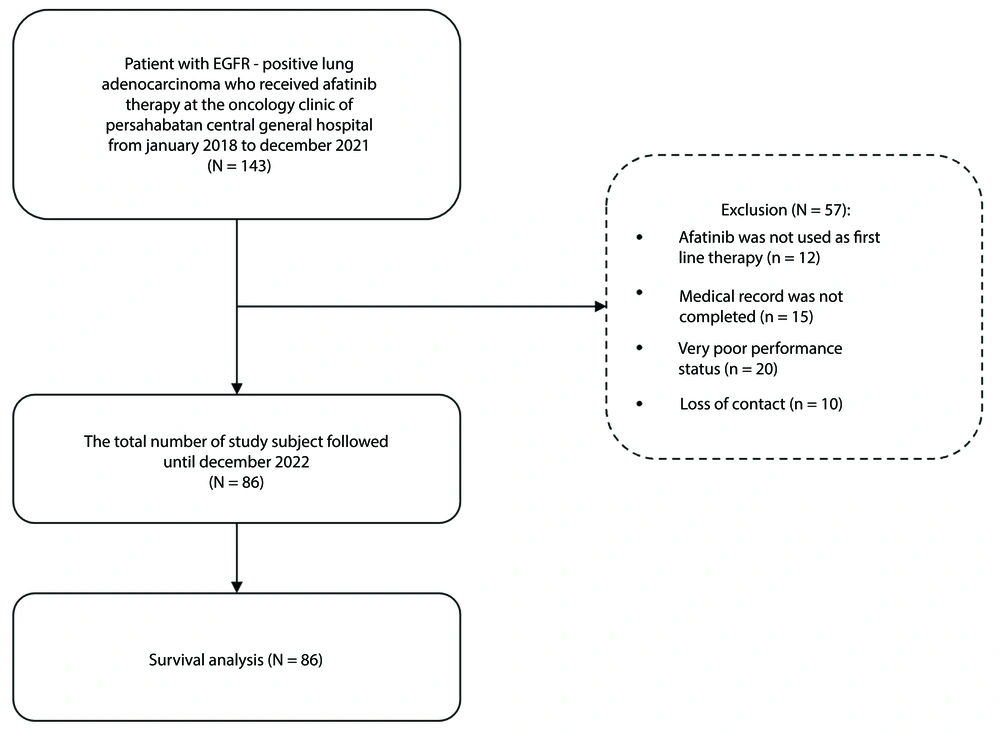
The study's findings indicate that among lung adenocarcinoma patients with EGFR mutations who received afatinib as first-line treatment at Persahabatan Hospital, the median PFS was 13 months (95% CI: 10.5 - 15.5), while the median OS was 17 months (95% CI: 14.9 - 19.1) (Figure 2). Additionally the one-year survival rate was 65.1%.
4.3. Analysis of Survival and Type of EGFR Mutation and Brain Metastasis
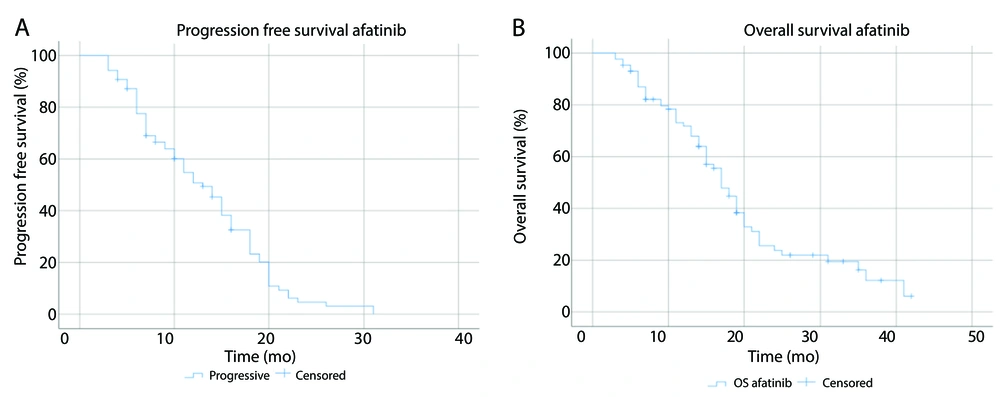
Based on the type of EGFR mutation, the median PFS for EGFR exon 19 mutation, exon 21 L858R mutation, and other mutations or combination were 15 months (95% CI: 11.5 - 18.5), 11 months (95% CI: 7.5 - 14.5), and 8 months (95% CI: 5.6 - 10.4), respectively (P = 0.18). The median OS was 19 months (95% CI: 14.7 - 23.3) for exon 19, 15 months (95% CI: 9.6 - 20.4) for exon 21 L858R, and 14 months (95% CI: 6.7 - 21.3) for other mutations or combination (P = 0.01). Kaplan-Meier curves for PFS and OS based on EGFR mutation types are presented in Figure 3A and B, while those based on brain metastasis are shown in Figure 3C and D.
Brain was the third most frequently site of metastasis found in the participants. This study obtained a median PFS of 11 months (95% CI: 6.3 - 15.7) for participants with brain metastasis and 14 months (95% CI: 11.6 - 16.4) for participants without brain metastasis (P = 0.6). The median OS of the participants with and without brain metastasis were both 17 months (95% CI: 11.8 - 22.2) and (95% CI: 13.6 - 20.4), respectively (P = 0.52).
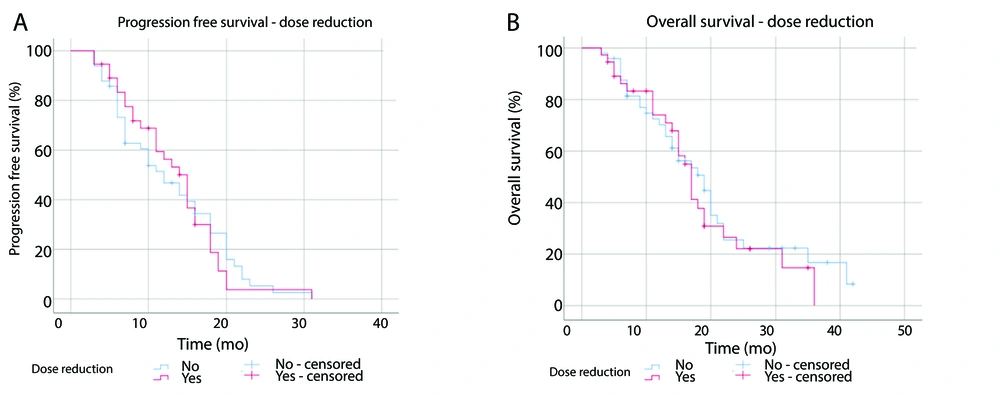
4.4. Analysis of Survival in Dose Reduction
Among the participants, 43% underwent afatinib dose reduction. The median PFS was 15 months (95% CI: 12.4 - 17.6) for the dose-reduction group and 12 months (95% CI: 8.1 - 15.9) for the non-reduction group (P = 0.85, Figure 4A). The median OS was 17 months (95% CI: 14.9 - 19.1) in the dose-reduction group compared to 19 months (95% CI: 13.7 - 24.3) in the non-reduction group (P = 0.59, Figure 4B).
4.5. Toxicity
The occurrence of anemia was found in 36 (42%) participants, with 22 (61%) having a history of anemia, 12 (33%) without a prior history, and 2 (6%) with an unknown history before the afatinib administration. The most frequently observed non-hematologic toxicities were diarrhea (81%), followed by skin rash (71%), stomatitis (70%), and paronychia (64%).
5. Discussion
The median PFS in this study was 13 months, aligning with prior findings 11 to 12.8 months (4-8). The median OS result in this study was 17 months, which differs significantly from other studies. Some studies have reported varying median OS results, ranging between 23.1 and 36.7 months (7-10). The lower median OS observed in this study may be attributed to the unavailability of subsequent EGFR-TKI treatments under the UHC program. Currently, the only available treatment for subsequent EGFR-mutated NSCLC under UHC is doublet chemotherapy, excluding bevacizumab and immunotherapy. Third-generation EGFR-TKIs, such as osimertinib, are not covered by UHC.
However, despite these limitations, the median OS reported in this study remains comparable to the PFS data from the AURA3 trial and the chemotherapy arm in other studies (11), suggesting that first-line afatinib still provides meaningful survival benefits in this patient population.
Real-world evidence suggests that modifying the afatinib dose can help reduce the occurrence and severity of adverse drug reactions while preserving its effectiveness. This emphasizes the importance of individualized dosing to improve treatment outcomes and guide clinical decisions (12). A study from Malaysia also showed significantly longer median PFS in exon 19 mutation group (16 months) compared to exon 21 L858R (8.7 months) or other mutations (9 months) (13). A meta-analysis study by Zhang et al. found that patients with exon 19 deletion significantly reduce the risk of progression compared to patients with exon 21 L858R mutation, although statistical significance was not observed. The hypothesis is that exon 19 deletion cause structural changes in EGFR, making TKIs or afatinib bind more tightly than in exon 21 L858R mutation. Additionally, the T790M mutation, which is associated to acquired EGFR-TKIs resistance, occurs more frequently in exon 21 L858R mutation. Exon 21 L858R mutation is also often found together with other uncommon mutations that may reduce their sensitivity to EGFR-TKIs (14). Our study identified a statistically significant difference in median OS among EGFR mutation types; 19 months for exon 19, 15 months for exon 21 L858R, and 14 months for other mutations (P = 0.01). The GIDEON study reported longer median OS for exon 19 mutation (33.9 months) compared to exon 21 mutations and other mutations (23.8 and 23.6 months). Other studies also demonstrated the superiority of afatinib in OS for exon 19 compared to exon 21 mutations (12, 15).
This study found a shorter median PFS in participants with brain metastasis, although statistically not significant, with 11 months compared to 14 months (P = 0.6). The GIDEON study found that the presence of brain metastasis does not affect response rates and disease control rates. The median PFS results from the GIDEON study are similar with our study; 10.5 months in patients with brain metastasis and 14.9 months in those without. The shorter PFS in brain metastasis group supports the negative prognostic impact (12, 16). The median OS value also did not show a significant difference, with each being 17 months (P = 0.52). This result aligns with a meta-analysis by Jin et al., which concluded that afatinib prolongs PFS in NSCLC patients with brain metastasis but does not affect OS. Another retrospective study found that only the EGFR-TKI group showed a superior benefit in intracranial PFS (17, 18). Patients with brain metastasis received benefit from afatinib similarly to those without brain metastasis, likely due to afatinib’s high concentration in cerebrospinal fluid and relatively-high penetration rate from plasma to cerebrospinal fluid (19, 20).
In this study, there was no statistically significant difference in survival between participants who underwent dose reduction (30 mg or 20 mg) and those who continued with the initial 40 mg dose, although the median PFS was longer in patients who underwent dose reduction (P = 0.85). Studies LUX-Lung 3, LUX-Lung 6, and LUX-Lung 7 also found that patients with afatinib dose reduction experienced longer PFS compared to those without dose reduction, although the difference was not statistically significant (20, 21). Longer median PFS values in participants who underwent dose reduction were also found in a retrospective study in Japan by Tanaka et al. (18.5 months compared to 7.9 months, P = 0.018) (22). A study by Chen et al. observed that patients using afatinib at a dose of 30 mg from the beginning of treatment had similar survival rate to patients using a dose of 40 mg (23). Additionally, plasma afatinib concentrations in patients who underwent a dose reduction to 30 mg were similar to patients who continued with the 40 mg dose (20). Dose adjustment according to tolerability does not affect the efficacy of afatinib. When the dose is optimal for each patient, clinical benefits are still achieved. Moreover, dose adjustments help to reduce the incidence and severity of afatinib-related toxicity, thereby decreasing the rate of therapy discontinuation due to side effects (21).
This retrospective study relied on secondary data sources (medical records), leading to the exclusion of certain subjects whose records were incomplete or already archived. Additionally, some evaluations such as response evaluation criteria in solid tumors (RECIST) could not be conducted timely every three months due to technical constraints, and hematological evaluations were not routinely performed. During the COVID-19 pandemic in 2020 - 2021, the diagnostic, therapeutic and patient evaluation processes were suboptimal, consequently affecting the number of subjects seeking treatment or undergoing RECIST evaluation.
5.1. Conclusions
Afatinib demonstrated efficacy for lung adenocarcinoma patients with EGFR mutations. Meanwhile, no statistically significant difference was found in PFS or OS survival rates between subjects receiving reduced doses of afatinib and those who did not. This is the first study to evaluate the efficacy and safety of afatinib under universal health coverage program in Indonesia.