1. Background
Lung cancer remains the most prevalent and deadly cancer globally (1, 2), with non-small cell lung cancer (NSCLC) accounting for around 85% of cases (3, 4). Approximately half of NSCLC patients are diagnosed at stage I-III (5), yet up to 60% of these patients experience disease relapse despite curative treatments (6). Distant metastases are found at the time of diagnosis in 30 - 40% of NSCLC patients, with bone, brain, liver, and adrenal glands as the most common metastatic sites (7, 8). Metastatic non-small cell lung cancer (mNSCLC) imposes a significant burden on patients, caregivers, healthcare systems, and society (9, 10). Importantly, several studies have highlighted the elevated prevalence of anaplastic lymphoma kinase (ALK) gene rearrangements in patients who eventually develop brain metastases (11, 12).
The ALK gene fusion is found in 3 - 5% of NSCLC and defines a distinct clinicopathologic subtype of NSCLC (13). Patients with ALK-positive NSCLC are typically younger than the general population of NSCLC patients and more likely to have never smoked or have a history of light smoking (14-16). The identification of ALK gene alterations has significant therapeutic implications. Patients with tumors harboring such rearrangements are highly sensitive to ALK inhibitors (ALKi) (17).
During the last decade, several ALKi for the treatment of ALK-positive NSCLC have been introduced into clinical practice in Italy. The Italian Medicines Agency (AIFA) has approved for reimbursement crizotinib in 2015, ceritinib in 2017, alectinib in 2018, brigatinib in 2020, and lastly, lorlatinib was approved in 2021 as a second-line option after front-line alectinib or following a sequence of crizotinib and another ALKi (18-22).
In the 2024 Clinical Practice Guidelines by the Italian Association of Medical Oncology (AIOM), next-generation ALKi inhibitors such as alectinib, brigatinib, and lorlatinib have been recommended as the preferred treatment options for patients with ALK-positive NSCLC. Ceritinib is suggested as another recommended intervention, while the first introduced crizotinib is considered useful in certain circumstances (23). Real-world evidence on the use of next-generation ALKi to treat ALK-positive NSCLC in Italy is currently limited.
2. Objectives
The present analysis exploited administrative databases of Italian healthcare to investigate the population of patients with ALK-positive NSCLC who were treated with ALKi. It aimed to describe patients’ characteristics (i.e., age, sex, comorbidities, presence of brain metastases), also according to the therapeutic sequences adopted, time to next treatment (TTNT), and mortality. Additionally, the study assessed healthcare resource utilization (HCRU) and the associated costs.
3. Methods
3.1. Study Design and Data Source
An observational retrospective analysis was conducted by integrating the administrative databases and pathological anatomy database of Local Health Units (LHUs) across Italy, including a population of approximately 6 million health-assisted individuals, with data available from January 2010 to June 2024. For the current study, Italian LHUs (representative of the Italian population) were selected based on their geographical distribution, data completeness, and the high-quality linked datasets.
The analysis used the following databases: (1) Beneficiaries’ database, which holds patients’ demographic data; (2) pharmaceuticals database, which records data on drug supplies through anatomical therapeutic code (ATC), prescription date, and number of packages; (3) hospitalization database, which collects hospitalization-related data, namely date of hospitalization, main and secondary diagnosis identified by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), and Diagnosis Related Group (DRG); (4) outpatient specialist service database, containing all data about laboratory tests, diagnostic procedures, and specialist visits; (5) payment exemption database, which contains data of the exemption codes that waive patients from the contribution charge for services/treatments when specific diseases are diagnosed. The dataset used consists solely of anonymized data. Approval was obtained from the ethics committees of the involved LHUs.
3.2. Study Population
From January 2020 to June 2024, mNSCLC patients were identified across all available periods based on the following criteria: (1) At least one prescription of anticancer drugs specific for mNSCLC (Appendix 1 in Supplementary File); OR (2) at least one prescription of anticancer drugs non-specific for mNSCLC (Appendix 2 in Supplementary File) AND at least one hospitalization with a main or secondary discharge diagnosis of lung cancer (ICD-9-CM codes: 162.2, 162.3, 162.4, 162.5, 162.8, 162.9, 235.7, 239.1, 195.1, 231.2); OR (3) at least one hospitalization with a main or secondary discharge diagnosis of lung cancer AND a subsequent diagnosis of metastasis (ICD-9-CM codes: 197, 198 or ATC code: M05BA08, M05BA03, M05BX04 [only minsan 041300]). Patients with at least one prescription of anticancer drugs specific for SCLC were excluded (Appendix 3 in Supplementary File).
Then, among the patients identified as mNSCLC according to the mentioned criteria, those who received a prescription specific for ALK-positive disease from 2020 were included in the analysis. The index date corresponded to the time of the first prescription of an ALKi. A 12-month period prior to the index date was used to describe patients’ previous history (characterization period). The follow-up period (at least 6 months) began at the index date and ended at the end of the study period, or the patient’s death, or exiting the database (whichever occurred first). Patients younger than 18 years, with previous cystectomies, with previous metastasis, or with less than 12 months of available data within the databases were excluded.
3.3. Patients’ Demographic and Clinical Characteristics
Age was recorded at the index date and presented as mean ± standard deviation (SD), while sex was reported as the proportion of males. The clinical characteristics were assessed using the Charlson Comorbidity Index (CCI), a scoring system based on 19 weighted comorbid conditions that may influence mortality risk (24). In this analysis, a modified version of the CCI, not accounting for the cancer score, was utilized. The concomitant diseases were detected by means of drug treatment and hospitalizations during the 12 months preceding the index date. The comorbidity index was reported as mean ± SD, and as the proportion of patients in each score.
3.4. Treatments
The treatments received by the mNSCLC patients — including chemotherapy (CT), immunotherapy, and targeted therapy — were identified across all available data periods in the study.
3.5. Time to Next Treatment and Overall Survival
The Kaplan-Meier method was applied to analyze TTNT, defined as the time (in months) from the initiation of treatment (index date) to the start of a subsequent line of therapy. Patients who did not progress to a subsequent line of therapy were censored at the date of database availability or death. Overall survival (OS) was defined as the time (in months) from the index date to the date of death. Survivors were censored at the date of database availability. The log-rank test was used to test the equality of survivor functions across treatments. Moreover, the Cox proportional hazards model was used as a multivariate approach to analyze the risk of switching to a subsequent treatment among different index treatments.
3.6. Evaluation of Healthcare Resource Consumption and Costs
Healthcare resource utilization and related costs in Euros (€) were evaluated during the 6 months of follow-up among the included patients (deaths and outliers excluded). The estimation focused on: (A) Drug treatments, including both oncological drugs and other comedications; (B) all-cause and cancer-related hospital admissions; (C) all-cause outpatient specialized services, namely laboratory tests, diagnostic procedures, and specialist visits. Cost analysis was carried out for all the included patients who were alive for at least 6 months after the index date.
3.7. Statistical Analysis
Continuous variables are presented as mean ± SD and median values, while categorical variables are expressed as numbers and percentages. A two-way ANOVA was conducted for the comparisons of HCRU across treatment groups. Kaplan-Meier curves with 95% confidence intervals (95% CI) were applied for TTNT and OS. The log-rank test was used to test the equality of survivor functions across treatments. Moreover, a Cox proportional hazards model was run as a multivariate approach to analyze the risk of switching to a subsequent treatment among different index treatments, with results presented as hazard ratios (HR) and corresponding 95% CI.
For the economic analysis, outliers, namely those costs exceeding three times the SD over the mean, were excluded. Since cost data are not expected to follow a normal distribution, a Generalized Linear Model (GLM) was used to evaluate the correlation between costs and the prescribed ALKi treatment, including age, gender, CCI, and prior brain metastasis as potential confounding variables. A gamma distribution with an identity link function was applied to ensure that the costs were not log-transformed, and the results were expressed in €. Post-estimation tests included residual analysis and checks for influential observations.
Following the “Opinion 05/2014 on Anonymization Techniques” drafted by the “European Commission Article 29 Working Party,” the results of subgroups of ≤ 3 patients were not reported (NR) for data privacy, as data might be potentially attributable to single individuals. A P-value < 0.05 was considered statistically significant, and all analyses were performed using STATA SE version 17.0 (StataCorp LLC, College Station, TX, USA).
4. Results
4.1. Identification of the Study Population
From a sample of 6,505,551 Italian citizens, 29,418 patients with mNSCLC were identified over the observation period from 2010 to 2023. The estimated incidence rate of mNSCLC in 2023 was 35.6 per 100,000 people/year. The number of prevalent cases of mNSCLC as of 31 December 2023 was 5,130 patients, and the number of new mNSCLC cases (incident) in 2023 was estimated to be 1,956. Among mNSCLC patients, 287 were ALK-positive (identified through ALKi prescription as a diagnosis proxy), corresponding to 5.6% of the prevalent mNSCLC population by the end of 2023. Additionally, from 2020 onwards, 8,642 patients with mNSCLC were identified, and 310 mNSCLC patients started a specific therapy for ALK-positive mNSCLC. Among the prevalent patients as of 31 December 2023, 5.6% received specific therapy for ALK-positive disease.
As shown in Table 1, a total of 274 ALK-positive mNSCLC patients were included and distributed as follows: Alectinib (N = 184), crizotinib (N = 50), brigatinib (N = 19), and CT plus ALKi (CT + ALKi, N = 16). Patients treated with lorlatinib (N = 4) and ceritinib (N < 4) were excluded from the analysis because of the small sample size. The proportion of males ranged from 48.9% in the alectinib group to 68.4% in the brigatinib group. Patients in the crizotinib-treated cohort were the oldest, and those in the brigatinib-treated cohort were the youngest (65.9 and 57.3 years, respectively). Age distribution showed notable differences, with alectinib and crizotinib having the highest proportions of patients aged 65 - 79 years (34.2% and 36.0%, respectively), while brigatinib had a higher proportion of younger patients (31.6% aged 35 - 49 years).
| Variables | Alectinib | Crizotinib | Brigatinib | CT + ALKi |
|---|---|---|---|---|
| Numbers | 184 | 50 | 19 | 16 |
| Gender (male) | 90 (48.9) | 25 (50.0) | 13 (68.4) | 9 (56.3) |
| Age (y) | 60.5 ± 12.8 | 65.9 ± 12.5 | 57.3 ± 16.3 | 64.2 ± 10.6 |
| 18 - 34 | 4 (2.2) | NI | NI | 0 (0.0) |
| 35 - 49 | 32 (17.4) | 4 (8.0) | 6 (31.6) | NI |
| 50 - 64 | 75 (40.8) | 19 (38.0) | 6 (31.6) | 7 (43.8) |
| 65 - 79 | 63 (34.2) | 18 (36.0) | 5 (26.3) | 6 (37.5) |
| ≥ 80 | 10 (5.4) | 8 (16.0) | NI | NI |
| CCI | 0.5 ± 0.7 | 0.7 ± 0.8 | 0.5 ± 0.6 | 0.6 ± 0.6 |
| 0 | 100 (54.3) | 23 (46.0) | 11 (57.9) | 8 (50.0) |
| 1 | 71 (38.6) | 20 (40.0) | 7 (36.8) | 7 (43.8) |
| ≥ 2 | 13 (7.1) | 7 (14.0) | NI | NI |
| Pre-index brain metastases | 17 (9.2) | 4 (8.0) | NI | 0 (0.0) |
| Post-index brain metastases | 17 (9.2) | 7 (14.0) | 0 (0.0) | NI |
| Follow-up in years | 2.4 ± 1.0 | 2.9 ± 1.0 | 1.8 ± 0.8 | 2.6 ± 1.0 |
| Characterization period | 9.1 ± 2.0 | 8.6 ± 1.8 | 10.4 ± 2.0 | 8.9 ± 1.8 |
Demographic and Clinical Characteristics of Patients with Non-small Cell Lung Cancer, Stratified by Anaplastic Lymphoma Kinase-Related Treatment a
The CCI, used to evaluate comorbidity burden, was comparable across groups, ranging from 0.5 (alectinib and brigatinib) to 0.7 (crizotinib). The alectinib-treatment group showed the largest proportion of patients with no major comorbidities (54.3% with CCI = 0), while crizotinib had the highest percentage of patients with a more severe comorbidity profile (14.0% with CCI ≥ 2). Brain metastases were found before the index date in 9.2% of alectinib-treated patients and 8.0% of crizotinib-treated patients, while there were no cases in the brigatinib and CT + ALKi cohorts. Brain metastases rose to 14.0% among crizotinib-treated patients during the post-index period and remained stable at 9.2% in the alectinib group. Follow-up duration was the longest for crizotinib (2.9 years) and the shortest for brigatinib (1.8 years). The characterization period was fairly consistent across groups, ranging from 8.6 to 10.4 years.
4.2. Treatment Pathways
The pattern of treatment pathways among 274 ALK-positive mNSCLC patients included in the analysis is described in Table 2. Alectinib was the most commonly used first-line therapy, prescribed to 67.2% (N = 184) of patients, followed by crizotinib to 18.2% (N = 50), brigatinib to 6.9% (N = 19), lorlatinib to 1.5% (N = 4), and CT combined with ALKi (CT + ALKi) to 5.8% (N = 16).
| First-Line Therapy (N = 274) | Second-Line Therapy (N = 103) |
|---|---|
| Alectinib (N = 184) | |
| CT + Alectinib (N = 20) | 10.9 |
| Lorlatinib (N = 19) | 10.3 |
| CT (N = 13) | 7.1 |
| CT + immunotherapy (N = 7) | 3.8 |
| Immunotherapy (N = NI) | 1.1 |
| Brigatinib (N = NI) | 0.5 |
| Ceritinib (N = NI) | 0.5 |
| Crizotinib (N = 50) | 22.0 |
| CT + Crizotinib (N = 11) | |
| CT (N = 6) | 12.0 |
| Alectinib (N = NI) | 4.0 |
| Immunotherapy (N = NI) | 4.0 |
| CT + immunotherapy (N = NI) | 2.0 |
| CT + lorlatinib (N = NI) | 2.0 |
| Brigatinib (N = 19) | 15.8 |
| CT + brigatinib (N = NI) | |
| Alectinib (N = NI) | 5.3 |
| CT + immunotherapy (N = NI) | 5.3 |
| Crizotinib (N = NI) | 5.3 |
| Lorlatinib (N = NI) | 5.3 |
| Lorlatinib (N = 4) | 25.0 |
| CT + immunotherapy (N = NI) | |
| Ceritinib (N = NI) | 0 |
| CT + ALKi (N = 16) | |
| CT (N = 8) | 50.0 |
| Alectinib (N = NI) | 6.3 |
Treatment Patterns in Metastatic Non-small Cell Lung Cancer Anaplastic Lymphoma Kinase-Treated Patients a
Of the patients who received first-line alectinib, 10.9% progressed to CT + alectinib as a second-line treatment, 10.3% to lorlatinib, 7.1% to CT alone, and smaller proportions to other regimens, including CT + immunotherapy (3.8%) and monotherapy with other immune-oncology agents (1.1%). For patients treated with first-line crizotinib, 22.0% received CT + crizotinib in the second line, while 12.0% received CT alone, and 4.0% in each group moved to either alectinib or immune-oncology agents.
Among those initially treated with brigatinib, 15.8% progressed to CT + brigatinib in the second line, while alectinib, CT + immunotherapy, crizotinib, and lorlatinib each accounted for 5.3% of second-line therapies. Patients receiving CT + ALKi as first-line therapy more commonly moved to CT alone (50.0%), with a small proportion switching to alectinib (6.3%).
4.3. Time to Next Treatment and Overall Survival
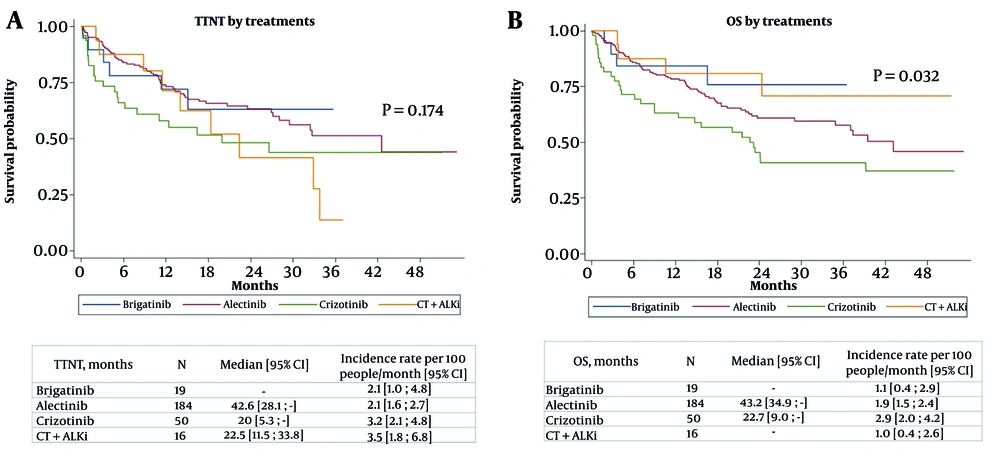
As depicted in Figure 1A, Kaplan-Meier analysis for TTNT showed no statistically significant differences between treatment groups (P = 0.174). Alectinib demonstrated the longest median TTNT at 42.6 months (95% CI: 28.1 - not reached), followed by CT + ALKi at 22.5 months (95% CI: 11.5 - 33.8), and crizotinib at 20 months (95% CI: 5.3 - not reached). Median TTNT for brigatinib was not reached. However, the incidence rate of treatment switches varied, with CT + ALKi having the highest rate at 3.5 events per 100 people-months (95% CI: 1.8 - 6.8), followed by crizotinib (3.2; 95% CI: 2.1 - 4.8), while alectinib and brigatinib both had lower rates at 2.1 events per 100 people-months (95% CI: 1.6 - 2.7 and 1.0 - 4.8, respectively).
The Cox regression model evaluating factors associated with the relative risk of treatment switching (Appendix 4 in Supplementary File) found no statistically significant associations. Compared to brigatinib, none of the other treatments had a significant effect on the risk of switching, with HR of 0.926 (95% CI: 0.394 - 2.18; P = 0.861) for alectinib, 1.466 (95% CI: 0.574 - 3.746; P = 0.424) for crizotinib, and 1.459 (95% CI: 0.507 - 4.197; P = 0.484) for CT + ALK agents. Sex did not influence the risk, with males showing an HR of 1.159 (95% CI: 0.772 - 1.74; P = 0.478). Age was also not significantly associated with switching, with HRs ranging from 1.034 (≥ 80 years; 95% CI: 0.363 - 2.943; P = 0.95) to 1.517 (65 - 79 years; 95% CI: 0.823 - 2.797; P = 0.182). Likewise, comorbidity burden, measured through the CCI, had no significant effect, with HRs of 1.379 (CCI = 1; 95% CI: 0.903 - 2.107; P = 0.137) and 1.399 (CCI ≥ 2; 95% CI: 0.675 - 2.9; P = 0.366). Lastly, the presence of previous brain metastases revealed no significant association with the risk of switching (HR 1.211; 95% CI: 0.599 - 2.447; P = 0.594).
Overall Survival was assessed using the Kaplan-Meier method (Figure 1B), and a significant difference was observed across treatment groups (P = 0.032). Alectinib showed the longest median OS at 43.2 months (95% CI: 34.9 - not reached), followed by crizotinib at 22.7 months (95% CI: 9.0 - not reached). For both brigatinib and CT + ALKi, median OS was not reached during the follow-up period. The incidence rate of OS events per 100 people-months was 1.9 (95% CI: 1.5 - 2.4) for alectinib, 2.9 (95% CI: 2.0 - 4.2) for crizotinib, and 1.1 (95% CI: 0.4 - 2.9) for brigatinib, with the CT + ALKi group having an incidence rate of 1.0 (95% CI: 0.4 - 2.6).
None of the treatments was significantly related to the risk of death: alectinib (HR 1.570; 95% CI: 0.564 - 4.372; P = 0.388), crizotinib (HR 2.165; 95% CI: 0.733 - 6.396; P = 0.162), and CT + ALKi (HR 0.931; 95% CI: 0.228 - 3.797; P = 0.921). Similarly, no association with mortality was found for sex (P = 0.279) and younger age groups (50 - 64 years: HR 1.409; 95% CI: 0.759 - 2.617; P = 0.278; 65-79 years: HR 1.651; 95% CI: 0.880 - 3.098; P = 0.119). However, subjects aged ≥ 80 years had a significantly higher risk of death (HR 3.673; 95% CI: 1.693 - 7.969; P = 0.001). Comorbidity Index (CCI = 1: HR 1.148; 95% CI: 0.762 - 1.729; P = 0.510; CCI ≥ 2: HR 1.219; 95% CI: 0.628 - 2.366; P = 0.559) and previous brain metastases (HR 1.745; 95% CI: 0.936 - 3.255; P = 0.080) were not significant predictors of death, although the latter showed a trend toward significance (Appendix 5 in Supplementary File).
4.4. Healthcare Resource Consumption and Costs
Healthcare resource utilization during the first 6 months of follow-up was assessed among alive patients (Table 3). The mean number of drug prescriptions per patient did not differ significantly across treatment groups (P = 0.804), ranging from 14.3 for brigatinib to 16.3 for crizotinib. The number of hospital admissions per alive patient was slightly higher in the crizotinib group (mean 0.5) compared to the alectinib (mean 0.3) and CT + ALKi groups (mean 0.1), though the differences were not statistically significant (P = 0.123). The number of outpatient specialized services varied significantly between groups (P = 0.002), with the CT + ALKi group showing the highest mean number of provisions per patient (14.3) and brigatinib the lowest (5.1).
| Number per Patient | Alectinib (N = 157) | Crizotinib (N = 35) | Brigatinib (N = 16) | CT + ALKi (N = 14) | P-Value |
|---|---|---|---|---|---|
| Drug prescriptions | 15.1 ± 7.6 | 16.3 ± 9.8 | 14.3 ± 8.3 | 15.3 ± 7.2 | 0.804 |
| Hospitalizations | 0.3 ± 0.7 | 0.5 ± 1.1 | 0.0 ± 0.0 | 0.1 ± 0.4 | 0.123 |
| Outpatient services | 9.0 ± 6.4 | 8.1 ± 5.6 | 5.1 ± 7.1 | 14.3 ± 10.2 | 0.002 b |
Average Number of Resources Per Alive Patient Attributable to Drugs, Hospitalizations, and Outpatient Service Prescriptions During 6-Month After Index Date (Outliers Excluded) a
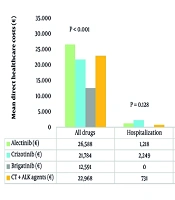
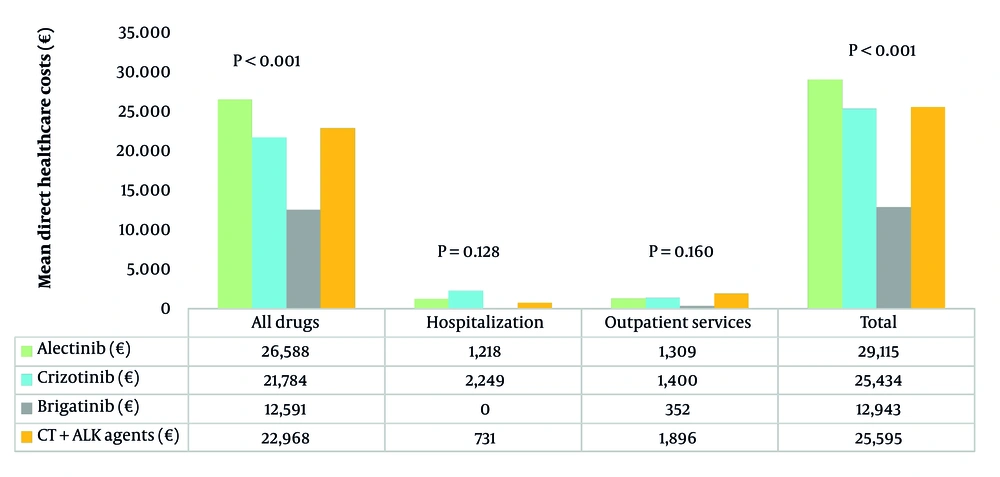
Total healthcare costs over 6 months per alive patient (Figure 2) were the highest for alectinib-treated patients (€29,115) and the lowest for brigatinib-treated patients (€12,943). Costs for all drug prescriptions (both cancer-related and unrelated) accounted for the majority of total expenses, with the highest values found among patients receiving alectinib (€26,588) and crizotinib (€21,784), while the brigatinib group showed the lowest drug-related costs (€12,591). Hospitalization costs were the highest for crizotinib (€2,249), while no patient on brigatinib required hospital access (€0). Outpatient service costs varied, with the CT + ALKi group incurring the highest expenses (€1,896) and brigatinib the lowest (€352).
The GLM analysis identified significant cost differences among treatments, using brigatinib as a reference (Appendix 6 in Supplementary File). Alectinib showed the highest incremental cost (€17,446; 95% CI €14,611 – €20,281; P < 0.001), followed by crizotinib (€14,534; 95% CI €10,509 – €18,558; P < 0.001) and CT + ALKi (€13,859; 95% CI €8,660 – €19,058; P < 0.001). Sex and age showed no significant impact on costs.
5. Discussion
This analysis provided an up-to-date picture of the ALK-positive mNSCLC population in Italian clinical practice, focusing on the epidemiology, baseline characteristics, treatment patterns, TTNT, OS, and economic burden of patients on ALKi therapy available during the study period. Considering a sample corresponding to 11% of the Italian national population, an incidence rate of mNSCLC of 35.6 per 100,000 people per year was estimated, in line with existing literature on lung cancer epidemiology in Italy. The Italian AIOM reported approximately 41,500 new lung cancer diagnoses in 2019, with NSCLC accounting for the majority of these cases (25).
Among the patients included in the analysis, the observed proportion of ALK-positive cases was 5.6%, slightly higher but still close to the range of 4% - 5% described in the international literature (26, 27). Such consistency suggests that the prescriptions of ALK inhibitors, as a proxy for ALK-positive NSCLC diagnosis, were a reliable approach for the effective identification of this patient subset (28).
The selection of first-line therapy for patients with ALK-positive NSCLC often considers factors such as age and comorbidity profiles. Thus, next-generation ALKi, like alectinib, ceritinib, and brigatinib, are becoming the preferred choice by clinicians compared to the first-generation ALKi crizotinib (29). The real-world data emerging here largely corroborate this view, as ceritinib, alectinib, and brigatinib were more commonly used as first-line therapy in patients with younger age and less severe comorbidity profiles over the crizotinib-treated cohort. During the study period, lorlatinib was not available as a first-line treatment.
The analysis of the treatment patterns and sequences showed that among ALK-positive mNSCLC patients, alectinib was the most commonly used first-line therapy in around two-thirds of the cases, followed by crizotinib, brigatinib, lorlatinib, and CT combined with an ALKi. Upon progression, various second-line treatments were observed. For alectinib-treated patients, common options included CT + alectinib and lorlatinib, while crizotinib-treated patients frequently switched to CT + crizotinib. Among brigatinib users, nearly 16% progressed to CT + brigatinib, while CT alone was the second-line option in half of those who initially received CT + ALKi.
These patterns, although variable, align with findings from available real-world analyses. Recent data of 463 ALK-positive NSCLC patients followed from July 2019 to March 2024 across 37 Italian centers showed that 82.5% of patients received alectinib as their first-line ALK inhibitor, underscoring its widespread adoption in clinical practice (30). Regarding second-line therapies post-alectinib, lorlatinib was the most commonly administered ALK inhibitor, accounting for 17% of cases, followed by brigatinib at 6% (29).
Kaplan-Meier survival analysis did not highlight significant differences in TTNT among treatment groups. Moreover, treatment type, sex, age, comorbidities, or brain metastases did not affect the likelihood of switching. When analyzing OS, significant differences emerged between treatment groups, with alectinib and crizotinib showing the longest median OS, while median OS for brigatinib and CT + ALKi was not reached.
In general, the observed OS outcomes are in line with findings from clinical trials comparing alectinib and crizotinib in ALK-positive NSCLC patients. In the phase III J-ALEX study, the final OS analysis did not show a significant difference between alectinib and crizotinib, likely due to treatment crossover. The 5-year OS rates were 60.9% for alectinib and 64.1% for crizotinib (31). Regarding brigatinib, the ALTA-1L trial demonstrated improved progression-free survival (PFS) compared to crizotinib in ALKi-naïve advanced ALK-positive NSCLC patients. Similar to our data, the median OS was not reached in either group, and no significant differences were detected in the HR for OS (32). To date, alectinib and brigatinib appear to provide significant clinical benefits in terms of PFS, although further research is needed to draw firm conclusions regarding OS.
The analysis of HCRU during the first 6 months of follow-up revealed comparable numbers of drug prescriptions across the treatment cohort. On the other hand, patients receiving the next-generation ALKi required fewer hospitalizations than those on first-line crizotinib therapy. Of note, the brigatinib-treated group showed by far the lowest consumption and costs for outpatient specialist services and no hospitalization expenses. Consistently, cost analysis showed that over the 6-month follow-up, total healthcare costs per alive patient were the lowest for those on brigatinib and the highest for alectinib-treated patients. A parallel trend was observed for all drug costs, which represented the most burdensome cost driver, with the highest values observed in the groups treated with alectinib and crizotinib.
A similar pattern was reported in previous studies that investigated the economic burden of treatments for ALK-positive NSCLC. A cost-effectiveness comparison in French ALK-positive NSCLC patients treated with ALKi in the first line found that alectinib had significantly higher drug-related costs compared to other ALKi inhibitors (33). In Italy, Ravasio et al. conducted a cost-utility analysis of brigatinib compared to alectinib in the treatment of ALK-positive NSCLC patients naïve to ALKi. The cost evaluation considered frontline therapies, subsequent therapies, best supportive care administration, comedications, adverse events, and health status. The results showed a quality-adjusted life-years (QALYs) increase of 0.216 and a cost reduction of €85,635 associated with brigatinib, indicating it as the most valid cost-utility option from the perspective of INHS (34).
The results of this analysis should be interpreted in view of some limitations. Given that the administrative databases are primarily conceived to track economic flows of reimbursable healthcare services and drugs, diagnoses could be identified only using proxies like hospitalization codes, exemption codes, and drug prescriptions. Likewise, the CCI was also determined by searching diagnoses with codes of drug prescriptions and hospitalizations; hence untreated/non-hospitalized comorbidities were not captured. In addition, the results were generated from a sample corresponding to 11% of the Italian population, which may limit their transferability on a larger national and international scale. Lastly, for some small subgroups of fewer than 3 patients, results could not be disclosed for privacy reasons, and this might limit the robustness of subgroup analysis. Furthermore, another limitation of this study was the small number of included ALK-positive NSCLC patients, and further studies on a larger sample should be conducted to corroborate the present findings.
5.1. Conclusions
In conclusion, this analysis provides valuable insights into the treatment of ALK-positive NSCLC in Italy before lorlatinib approval as first-line therapy, highlighting trends in epidemiology, treatment patterns, survival outcomes, and the associated economic burden. The findings support the reliability of ALKi prescriptions to identify ALK-positive cases within the administrative database and confirm a shift towards next-generation ALKi, such as alectinib, ceritinib, and brigatinib, as first-line treatments.
The analysis of HCRU revealed that next-generation ALKi were associated with fewer hospitalizations than crizotinib. Alectinib was found to have the highest treatment costs (€29,114.8), primarily due to drug expenses, while brigatinib was confirmed to be the most cost-effective option (€12,942.6), in line with existing evidence. Further research and real-world data are necessary to optimize treatment strategies and clinical outcomes in the population of ALK-positive NSCLC patients.