1. Background
Breast cancer is the leading cause of cancer-related mortality in women. Following surgical and/or systemic treatment, a large number of patients require radiotherapy as an adjuvant treatment. Radiotherapy plays an important role in breast cancer treatment since it decreases the risk of local recurrence and increases the patients’ survival (1-4). The adverse effects of radiotherapy, particularly on organs at risk (OARs), such as the lung or thyroid, cannot be fully avoided. Several patients with breast cancer, especially those with non-metastatic disease, tend to have long survival times, which predisposes them to develop the side effects of the treatment (5, 6).
There are concerns about the radiotherapy-related occurrence of secondary malignancies in organs exposed to radiation in breast cancer survivors (7-10). The incidence of secondary malignancies following oncological treatment for a primary cancer was previously underestimated due to shorter follow-ups and worse prognosis of the patients. However, with improved survival rates, it has now become a significant clinical concern. The cumulative occurrence of secondary malignancies following radiation therapy reaches up to 20 percent, especially in women younger than 40 years old (11, 12).
A retrospective study on more than 980 patients with breast cancer from three cancer research centers in Tehran, Mashhad, and Isfahan from Sep 1995 to Sep 2010 found 94 cases with second primary neoplasms. However, they concluded that the risk of developing a second cancer was more dependent on genetic and environmental factors that caused the primary cancer, rather than being dependent on the type of treatment (13).
Intensity-modulated radiation therapy (IMRT) and volumetric-modulated arc therapy (VMAT) techniques have recently been utilized to improve dose homogeneity in breast cancer treatment. However, compared to the three-dimensional method, these new radiotherapy techniques have a higher low-dose volume for organs near the field of treatment, including the contralateral breast and both lungs, which might increase the risk of radiation-induced malignancies (14-17).
According to two large cohort studies, secondary malignancies after breast radiation mainly arise in organs proximal to the treatment zone (18, 19). Epidemiological research during the last two decades failed to accurately determine the risk of secondary malignancies. Given the improvements in long-term survival of patients with breast cancer and the fact that nearly 80% of secondary cancers originate in or near the radiation field, the risk of radiation-induced secondary malignancies has drawn a great deal of attention in recent years (16, 20).
Based on dose-volume histogram (DVH) curves and equivalent doses, several biological models have assessed the risk of secondary cancers following radiotherapy. Schneider's organ-equivalent dose (OED) can estimate the absolute increased risk of secondary cancers after radiotherapy exposure based on data from atomic bomb survivors and patients with Hodgkin's lymphoma who were treated with radiotherapy (20-24).
2. Objectives
In this study, DVHs and different models were employed to determine the risk of secondary malignancies following breast radiotherapy using the three-dimensional conformal method. The aim of this study was to determine the amount of dose received by OARs and the probability of secondary malignancies.
3. Methods
3.1. Study Design and Sample Size
This study was a cross-sectional observational study. It was confirmed by the Local Committee of Medical Ethics, Shahid Beheshti University of Medical Sciences (approval code: IR.SBMU.MSP.REC.1399.577).
Forty female patients with breast cancer who had been treated at the radiation oncology department of Imam Hossein Hospital were assessed.
Exclusion criteria included bilateral breast cancer, breast implants, a history of surgery or breast cancer or radiotherapy on the contralateral breast, and a history of previous radiotherapy to the chest wall.
All patients underwent computerized tomography (CT) simulation (a special CT scan that is performed for radiotherapy planning) according to the protocol of the department, with a supine position on the breast board with the ipsilateral arm above the head and 5 mm CT slices.
Without making any changes in the plans, we checked the accuracy of the contouring of OARs, including both lungs, heart, thyroid, contralateral breast, spinal cord, and esophagus. Afterward, the dose received by each organ was calculated by using a formula and a DVH. In case of the presence of any organ overdose, the treating physician was informed; however, our calculations were done based on the original data. The radiation oncologist approved the treatment plan based on the DVH curves, without any intervention from the researchers.
3.2. Patients’ Characteristics
We recorded the patient and treatment characteristics, including age, stage of the disease, disease sidedness, type of surgery, the presence of shield, treatment energy, presence of supraclavicular fields, and prescribed dose.
3.3. Assessment of the Probability of Secondary Malignancy
All patients were planned by Isogray software version 4.2.3 and were treated with three-dimensional conformal radiotherapy (3D-CRT). For this study, the doses received by the affected organs, including the average dose, minimum dose V5 [the volume of the organ that receives 5 grays (Gy)], V20, V10, and V30, were measured.
To assess the probability of secondary malignancy in the contralateral breast and both lungs, biological models provided by the Committee on Biological Effects of Ionizing Radiation (BEIR VII) were used. The concept of OED, which takes into account the effect of treatment session (fractionation), repair, and repopulation parameters, was used to estimate and compare the risk of secondary malignancies after radiotherapy. According to the concept of OED, two different radiotherapy plans that have the same risk of secondary malignancy have the same OED.
OEDs for the opposite breast and lungs were calculated based on the DVH curves using a linear dose-response relationship with exposure dose as follows (equivalent dose; Equation 1):
The possibility of cell death rises by increasing the dose, and as a result, the risk of cancer decreases due to the death of mutated cells (Equation 2):
Assuming that there would be a balance between cell death and cell recovery, the dose-response (fractionated scheme) is expected to reach a plateau after a linear increase up to a certain dose (Equation 3):
Finally, by considering the number of treatment sessions, this formula can be used (Equation 4):
The aforementioned formulas define V0 as the total volume of the organ, VDi as the volume of the organ that is exposed to the radiation dose Di, and α and R as the specific parameters of each organ. These parameters have been derived from data obtained from atomic bomb survivors and Hodgkin's patients who had been treated with radiotherapy.
3.4. Assessment of Absolute Risk Excess
To estimate the risk of developing a secondary malignancy, the excess absolute risk (EAR) was calculated. The EAR represents the absolute difference in the malignancy incidence between individuals exposed to a radiation dose (d) and those unexposed, expressed per Gy over a specific number of years in a population of 10,000 individuals.
The parameters used in this formula were obtained from Schneider's model. In this model, "age-x" refers to the patient's age at the time of radiation exposure, while "age-a" indicates the age the patient is expected to reach. For the purposes of our calculations, we assumed an expected age of 70 years, based on previous studies (Equation 5).
3.5. Statistical Analysis
Statistical analysis was performed using SPSS software version 22. The normal distribution of quantitative data was checked using the Shapiro-Wilk test, and comparisons were made using the t-test. Mean and standard deviation was used to describe quantitative data with a normal distribution, while the median and interquartile range were used to describe quantitative data with a non-normal distribution. Qualitative data were expressed as frequencies and percentages. Tables and graphs were utilized to present the data. The level of statistical significance was set at P-value < 0.05.
4. Results
4.1. Demographic and Basic Clinical Information
The mean ± SD age of the patients was 53.38 ± 11.94 years (28 - 80 years). Twenty-five (62.5%) patients had left-sided and 15 (37.5%) had right-sided breast cancer. Twenty-four (60%) patients had undergone breast conserving surgery (BCS) while the other 16 (40%) had been treated with modified radical mastectomy (MRM). Fourteen (35%) of the patients were treated with a low-energy machine (6 MW), and 26 (65%) were treated with a high-energy machine (6 and 18 MW). All patients received treatment for breast and/or chest wall, and among them, 31 (77.5%) received treatment including fields for lymph nodes (all with supraclavicular fields). All patients received a total dose of 50 Gy in 25 fractions, and patients with preserved breasts received an additional boost dose of 12 - 14 Gy in 5 - 7 fractions.
4.2. Analysis of Dose-Volume Curves
The results showed that patients treated with supraclavicular fields received a mean dose of 0.4 ± 0.22 Gy to the contralateral breast, which was marginally lower than the mean dose of 0.43 ± 0.21 Gy received by patients treated without supraclavicular fields. This difference may be explained by the use of larger tangential fields (i.e., high tangent fields) in patients who did not receive supraclavicular irradiation. In the latter group, the V5 was 0.
Patients who had supraclavicular fields received a mean dose of 0.67 ± 0.30 Gy to the contralateral lung, which was significantly higher than that of those who did not have this field (0.30 ± 0.22 Gy), and V10 was also 0.
Regarding the ipsilateral lung, patients who were treated with supraclavicular fields received a mean dose of 18.2 ± 2.95 Gy, compared to 11.39 ± 4.61 Gy in patients who did not have supraclavicular fields (Table 1).
| Involved Organ; Dose | All Patients | Supraclavicular + Tangential Fields | Only Tangential Fields |
|---|---|---|---|
| Contralateral breast | |||
| Maximum dose (Gy) | 6.00 ± 4.95 | 6.44 ± 5.48 | 4.46 ± 1.8 |
| Average dose (Gy) | 0.4 ± 0.22 | 0.4 ± 0.22 | 0.43 ± 0.21 |
| Contralateral lung | |||
| Maximum dose (Gy) | 5.47 ± 6.80 | 6.15 ± 7.59 | 3.11 ± 1.27 |
| Average dose (Gy) | 0.59 ± 0.32 | 0.67 ± 0.3 | 0.30 ± 0.22 |
| V5 (%) | 0.09 ± 0.57 | 0.12 ± 0.64 | 0 |
| Ipsilateral lung | |||
| Maximum dose (Gy) | 56.33 ± 6.50 | 56.28 ± 6.28 | 56.52 ± 7.61 |
| Average dose (Gy) | 16.66 ± 4.40 | 18.20 ± 2.95 | 11.39 ± 4.61 |
| V5 (%) | 56.31 ± 13.95 | 61.82 ± 7.94 | 37.32 ± 13.75 |
| V10 (%) | 40.30 ± 10.92 | 44.46 ± 6.74 | 25.97 ± 10.63 |
| V20 (%) | 30.92 ± 8.83 | 34.12 ± 5.83 | 19.89 ± 8.70 |
| V30 (%) | 26.67 ± 8.10 | 24.49 ± 5.63 | 16.98 ± 8.02 |
Abbreviation: Gy, grays.
a Values are expressed as mean ± SD.
Table 2 demonstrates the average dose received by the thyroid gland, heart, esophagus, and spinal cord. As it shows, patients treated with supraclavicular fields received significantly higher doses to the thyroid gland compared to others (16.33 ± 6.88 Gy and 1.44 ± 0.28 Gy, respectively).
| Involved Organ; Dose | All Patients | Supraclavicular + Tangential Fields | Only Tangential Fields |
|---|---|---|---|
| Thyroid gland | |||
| Maximum dose (Gy) | 37.66 ± 20.73 | 47.98 ± 8.34 | 2.12 ± 0.62 |
| Average dose (Gy) | 12.98 ± 8.72 | 16.33 ± 6.88 | 1.44 ± 0.28 |
| V5 (%) | 37.85 ± 24.51 | 48.48 ± 15.05 | 0 |
| V10 (%) | 30.22 ± 20.84 | 39.00 ± 14.52 | 0 |
| V20 (%) | 24.29 ± 19.63 | 31.34 ± 16.52 | 0 |
| V30 (%) | 19.81 ± 18.50 | 25.57 ± 17.11 | 0 |
| Esophagus | |||
| Maximum dose (Gy) | 28.81 ± 18.17 | 36.60 ± 12.25 | 1.96 ± 0.62 |
| Average dose (Gy) | 4.32 ± 3.41 | 5.32 ± 3.24 | 0.86 ± 0.42 |
| V5 (%) | 17.42 ± 13.58 | 22.47 ± 11.05 | 0 |
| V10 (%) | 10.34 ± 11.16 | 13.34 ± 10.98 | 0 |
| V20 (%) | 6.51 ± 8.65 | 8.39 ± 9.00 | 0 |
| V30 (%) | 4.07 ± 6.60 | 5.25 ± 7.08 | 0 |
| Spinal cord | |||
| Maximum dose (Gy) | 22.73 ± 16.08 | 30.26 ± 11.85 | 1.26 ± 0.38 |
| Average dose (Gy) | 2.68 ± 2.31 | 3.37 ± 2.18 | 0.31 ± 0.21 |
| V5 (%) | 11.97 ± 9.37 | 15.45 ± 7.66 | 0 |
| V10 (%) | 7.46 ± 8.25 | 9.62 ± 8.18 | 0 |
| V20 (%) | 4.60 ± 6.35 | 5.94 ± 6.65 | 0 |
| V30 (%) | 2.20 ± 4.45 | 2.84 ± 4.88 | 0 |
| Heart in patients with left breast cancer | |||
| Maximum dose (Gy) | - | 52.99 ± 4.88 | 52.01 ± 5.76 |
| Average dose (Gy) | - | 7.58 ± 1.34 | 5.85 ± 1.38 |
| V5 (%) | - | 23.99 ± 6.35 | 19.88 ± 6.02 |
| V10 (%) | - | 15.16 ± 3.52 | 10.09 ± 2.36 |
| V20 (%) | - | 11.57 ± 2.36 | 7.59 ± 1.95 |
| V30 (%) | - | 9.13 ± 2.25 | 6.16 ± 1.80 |
| Heart in patients with right breast cancer | |||
| Maximum dose (Gy) | - | 9.90 ± 9.16 | 9.31 ± 8.35 |
| Average dose (Gy) | - | 1.20 ± 0.50 | 1.45 ± 0.54 |
| V5 (%) | - | 1.30 ± 2.11 | 2.52 ± 3.56 |
| V10 (%) | - | 0 | 0 |
| V20 (%) | - | 0 | 0 |
| V30 (%) | - | 0 | 0 |
Abbreviation: Gy, grays.
a Values are expressed as mean ± SD.
In patients with left breast cancer, those who underwent supraclavicular radiation received higher doses to the heart compared to others (7.58 ± 1.34 Gy and 5.85 ± 1.38 Gy, respectively). In addition, supraclavicular fields made the esophagus receive higher doses of radiation during treatment (5.32 ± 3.24 Gy compared to 0.86 ± 0.42 Gy). Furthermore, in patients with supraclavicular fields, the spinal cord received a higher mean dose (3.37 ± 2.18 Gy compared to1.26 ± 0.38 Gy). Patients treated with a supraclavicular field also received a maximum dose of 30.26 ± 11.85 Gy.
4.3. Evaluation of the Risk of Secondary Malignancy
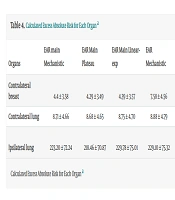
Based on Schneider's OED and EAR biological formulas, we calculated the average EAR for the opposite breast. The results showed that the average EAR, using all three biological models considering the patients’ actual age at the time of radiotherapy (EAR main), was 4.34. However, when considering radiation exposure at the age of 30 and a life expectancy of 40 additional years (i.e., up to the age of 70), the average EAR increased to 7.746. This indicates an absolute difference in the incidence of malignancy in individuals exposed to this dose compared to those who have not been exposed, per 10,000 person-years. For the lung on the treatment side, the calculated numbers were 224.12 and 229.00, respectively, while for the opposite side, they were 8.71 and 8.89, respectively. The calculated OED and EAR for each organ are represented in Tables 3 and 4.
| Organs | OED Mechanistic | OED Plateau | OED Linear-exp |
|---|---|---|---|
| Contralateral breast | 0.93 ± 0.56 | 0.90 ± 0.54 | 0.92 ± 0.56 |
| Contralateral lung | 1.11 ± 0.60 | 1.11 ± 0.59 | 1.12 ± 0.60 |
| Ipsilateral lung | 28.6 ± 9.32 | 27.9 ± 9.24 | 29.35 ± 9.79 |
Abbreviation: OED, organ-equivalent dose.
a Values are expressed as mean ± SD.
| Organs | EAR Main Mechanistic | EAR Main Plateau | EAR Main Linear-exp | EAR Mechanistic | EAR Plateau | EAR Linear-exp |
|---|---|---|---|---|---|---|
| Contralateral breast | 4.4 ± 3.58 | 4.29 ± 3.49 | 4.39 ± 3.57 | 7.58 ± 4.56 | 7.38 ± 4.45 | 7.54 ± 4.55 |
| Contralateral lung | 8.71 ± 4.66 | 8.68 ± 4.65 | 8.75 ± 4.70 | 8.88 ± 4.79 | 8.86 ± 4.74 | 8.93 ± 4.79 |
| Ipsilateral lung | 223.20 ± 72.24 | 218.46 ± 70.87 | 229.78 ± 75.01 | 229.10 ± 75.32 | 223.22 ± 73.96 | 234.79 ± 78.28 |
Abbreviation: EAR, excess absolute risk.
a Values are expressed as mean ± SD.
4.4. Comparison Between Patients Treated with the Elekta or Varian Machine
The study revealed that there was a statistically significant difference in the average dose received by the contralateral breast between patients treated with Elekta and Varian. Patients treated with Elekta (with 6 MV energy) received an average dose of 0.59 ± 0.19 Gy, while patients treated with Varian (with 18 MV energy) received an average dose of 0.15 ± 0.3 Gy (P < 0.001). Other parameters for the opposite breast regarding comparison of Electa and Varian are shown in Table 5. For other investigated organs, there was no significant difference between the two machines (P > 0.05).
| EAR; Devise | Mean ± SD | P-Value |
|---|---|---|
| EAR mechanistic | ||
| Elekta | 11.4 ± 4.6 | < 0.001 |
| Varian | 5.28 ± 2.43 | |
| EAR plateau | ||
| Elekta | 11.37 ± 4.62 | < 0.001 |
| Varian | 5.23 ± 2.47 | |
| EAR linear-exp | ||
| Elekta | 11.62 ± 4.73 | < 0.001 |
| Varian | 5.35 ± 2.53 | |
| EAR main mechanistic | ||
| Elekta | 6.96 ± 4.00 | 0.002 |
| Varian | 2.89 ± 2.1 | |
| EAR main plateau | ||
| Elekta | 6.94 ± 3.99 | 0.002 |
| Varian | 2.87 ± 2.18 | |
| EAR main linear-exp | ||
| Elekta | 7.09 ± 4.08 | 0.002 |
| Varian | 2.93 ± 2.23 |
Abbreviation: EAR, excess absolute risk.
5. Discussion
In most centers, 3D-CRT is employed for breast cancer treatment, as it enhances dose uniformity to the target tumor volume (25).
Approximately 80% of secondary malignancies developed in or near the radiotherapy field, making the risk of radiation-related malignancies an important concern, especially in younger patients (17, 20, 26). However, due to the lack of long-term follow-up and epidemiological data, estimates of this risk remain imprecise.
To address this issue, several biological models have been developed using data from nuclear warfare survivors and patients with Hodgkin's disease treated with radiotherapy (20, 24, 27). In our study, the EAR for the ipsilateral lung was higher than that of similar studies (20, 21, 28-30).
Previous studies have shown that the risk of radiation-induced pneumonitis is dependent on the V20 parameter of the lung (28). In our study, V20 ranged from 21.85% to 47.33% for patients treated with a supraclavicular field and from 9.64% to 39.11% for those treated without a supraclavicular field, showing that some patients have received higher than standard radiation doses. The difference between the three used biological models was small, which is consistent with previous studies (20, 21).
Protecting the contralateral breast from radiation exposure is essential in breast radiotherapy treatment planning. In our study, the average dose to the contralateral breast was 0.27 Gy, which is in line with or even lower than the previous studies (21, 31, 32).
For the contralateral lung, the mean dose and V5 were 0.61% and 0.36%, respectively. These findings are also similar to previous studies (21).
The results of our study indicate that the three-dimensional method of radiotherapy treatment results in appropriate protection of adjacent organs from radiation.
In our center, the comparison of DVH between two Elekta linear accelerators with a single energy of 6 MW and Varian with energies of 6 and 18 MW showed that all organs except the contralateral breast received higher doses of radiation with Varian, probably due to the differences in photon energies.
Our study found that V20 for the treated lung and heart in patients with left breast cancer with supraclavicular fields was slightly more than acceptable, indicating a need for more accurate treatment planning. Radiation doses to other organs were within acceptable limits.
It should be pointed out that this study did not report the actual incidence of secondary cancers in patients. Instead, it estimated the theoretical probability of secondary cancer occurrence based on radiation dose calculations. The patients were not followed up over a long period, and the incidence of malignancies was not directly observed. The data presented suggest the incidence of secondary cancer based on available models and theoretical dose calculations. However, the extent to which these theoretical estimations correspond to real-world clinical outcomes remains uncertain and requires long-term clinical follow-up studies with actual patient data to validate these models.
Epidemiological studies have shown that there is a risk of secondary cancers following radiotherapy, but the precise magnitude of this risk and its direct correlation with the radiation dose to each organ need to be supported by longitudinal clinical data. This study did not provide such follow-up, nor was it the primary aim.
5.1. Conclusions
This study further highlights the importance of precise treatment planning to minimize the complications of OARs and optimize patient outcomes. Especially, more care must be taken in contouring and planning of supraclavicular fields in order to minimize the radiation to the lungs and heart. In addition, patients should be closely monitored for potential complications in long-term follow-ups, and appropriate interventions should be taken promptly.