1. Background
Breast cancer is one of the most common causes of death in women all around the world. It is estimated that more than 1.38 million new women suffered from breast cancer (1). Anti-estrogen therapy is the choice strategy for estrogen receptor positive breast cancer patients. However, a large number of breast cancers do not respond to this type of treatment because of lack of estrogen receptor. Furthermore, another group of breast cancer becomes insensitive to hormone therapy after first therapy and immediately promotes to grow (2). Although chemotherapy is an alternative strategy for treating insensitive and metastatic breast cancer, but many of the treated cancers often develop a recurrence. Therefore, innovating novel agents is an urgent requirement for attenuating the mortality rate (3). The receptor tyrosine kinase like orphan receptor 1 (ROR1) is a transmembrane protein and belongs to the receptor tyrosine kinase family (4). This protein has five domains, three extracellular including an immunoglobulin like motif, frizzled and Kringle domains, transmemebrane part and an intracellular tyrosine kinase domain (5). Over expression of ROR1 is found in the embryonic stage (6) and several cancers including B-CLL (7), B-ALL (8), gastric carcinoma, non-small cell carcinoma cell lines (9) and breast cancer (10). Zhang et al. revealed the overexpression of ROR1 in the breast cancer related with lacked of HER2/neu and hormone receptors. Moreover, upregulation of ROR-1 in primary breast cancer is associated with poor differentiation and shorter survival rate (10). There are limited or lack of ROR-1 in normal cells while over expression of this protein has been reported among human cancer cells, therefore ROR1 may be applied as a potent target for immunotherapy.
Staphylococcal enterotoxin B (SEB), a 28 KDa superantigen, is a powerful T cell activator. This protein binds to MHC class II on antigen presenting cells (APCs) and then forms complex with the variable region of β chain of T cell receptor. The binding site of SEB on the APCs has differed from that of specific antigens (11). SEB exerts a potent mitogenic effect on both CD4+ and CD8+, increasing cytokines including interferon-γ (INF-γ), interleukin 2 (IL-2), and tumor necrosis factor-α (TNF-α), finally promotes a powerful antitumor immunity (12).
One of the main goals of immunotherapy against tumor is to create a specific tumoral antigen response that participates to the tumor eradication. Designing the combined construct, enabling to activate both cellular and humoral anti-tumor immunity is an effective therapeutic method which restricts or eradicates tumor progression. To date, it is possible to design a suitable combined construct, based on B cell and the T cell epitope map using bioinformatics methods.
2. Objectives
Here, we purposed to design the immunotherapeutic target constructed having two parts. The first one was extracellular domains of ROR1 as specific tumoral antigens for inducing B cell lymphocyte and second one is SEB, an adjuvant to create anti-tumoral response via T cell lymphocyte.
3. Materials and Methods
3.1. Protein Sequences and Designing the Construct
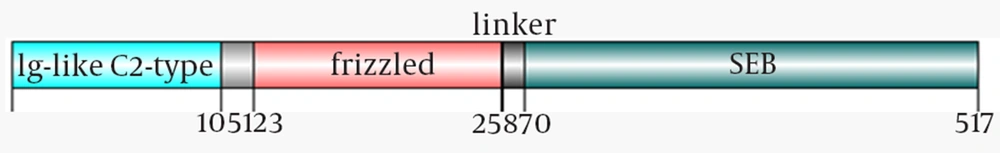
To obtain the protein sequences of ROR-1 and SEB, the UniProtKB database was used. The accession number of SEB and ROR-1 was P01552 and A2VCQ3, respectively. To make a fusion protein based on ROR-1 and SEB having robust specific anti-tumoral activity, the extracellular part of ROR-1 containing the frizzled domain and Ig-like C2 type domain was selected and joined to the complete sequence of SEB with GSGGSGGSGGSG as a hydrophobic amino acid linker. To survey the antigenicity of designed construct, the online database VaxiJen v2.6 was utilized.
3.2. The Physico-Chemical Characteristics
The physiochemical features, theoretical isoelectric point (pI), molecular weight, total number of positive and negative residues, extinction coefficient, instability index, aliphatic index and grand average hydropathy of recombinant construct were analyzed by the Expasy ProtParam server (http://us.expasy.org/tools/protparam.html).
3.3. Prediction of Secondary Structure
To assess the secondary structure of ROR-1-SEB fusion protein, the online database GOR IV (13) were performed. To confirm findings from Gor4, fusion proteins evaluated by PHDsec (https://www.predictprotein.org).
3.4. Prediction of 3D structure
The 3D model of the designed construct, was evaluated by the I-TASSER online server (14). To compute the Energy minimization and Ramachandran plot of the suggested 3D model, Swiss-PdbViewer and Procheck server (15) were executed.
3.5. Prediction of B-Cell and T-Cell Epitopes
To predict the linear and conformational B-cell epitopes, full-length primary sequences of designed fusion protein were computed using BCPreds (16) and web server CBTOPE (17), respectively. In addition, for prediction of discontinuous B-cell epitopes from three-dimensional protein structures, Discotope server was employed (18). Furthermore, to identify the antigenicity of selected BCPreds epitopes with the cutoff value of > 0.8, the VaxiJen (threshold = 0.4, ACC output) was used. On the other hand, BCPreds software was utilized for predicting continuous B cell epitopes based on different parameters including hydrophilicity, plasticity, exterior accessibility, antigenicity, flexibility, surface exposed, and polarity along full length designed construct. To survey MHC Class I and MHC Class II binding common epitopes, Propred-1 (47 MHC Class I alleles) (19) and Propred (51 MHC Class II alleles) (20) servers was exerted, respectively. In accordance with two mentioned servers, the whole numbers of MHC allele interaction were estimated. The antigenicity value of predicted epitopes was analyzed by VaxiJen.
3.6. Prediction of Allergenic Regions
To recognize the possibility of existence of allergenic regions, AlgPred was used. The server predict allergens along the fusion protein sequence in accordance with similarity to known epitopes. Next the allergenicity was evaluated through SDAP database (21).
3.7. Prediction of Protein Solubility
To assess the protein solubility, the recombinant protein solubility prediction was exerted (22).
4. Results
4.1. Designing the Construct
The extracellular part of ROR1 was selected for designing the N-terminal of chimeric because of its accessibility to antigen presenting cells. This part has consisted of the Frizzled domain, which involves in proliferation, cell polarity and cell developing, and Ig-like C2 type domain, which participates in cell-cell recognition and immune system stimulation. Full- length of SEB was conjugated with GSGGSGGSGGSG linker to ROR1 and formed C-terminal of the described construct. Figure 1 depicts the schematic diagram of chimeric construct using DOG 1.0 software (23). Based on VaxiJen outcomes, the antigenicity index of ROR1 fragment alone, SEB fragment, linker and the combination of all three mentioned parts were 0.6488, 0.5618, 4.9849 and 0.5994.
4.2. The Physico-Chemical Characteristics
Our construct has 517 amino acids consisting 65 negatively charged residues and 62 positively charged residues. Its molecular weight was 59.0199 KDa. The value of pI was 6.42 that indicated the acidity feature of the designed construct. The extinction coefficient of ROR1-SEB was 58,065 M-1 cm-1 at 280 nm. The appraised half-life was > 10. In accordance with an instability index of ExPASy, ProtParam (< 40), ROR1-SEB was categorized as an unstable protein (instability index=41.90). The alphabetic index and Grand average of hydropathicity of ROR1-SEB was 70.48 and -0.527, respectively.
4.3. Prediction of Secondary Structure
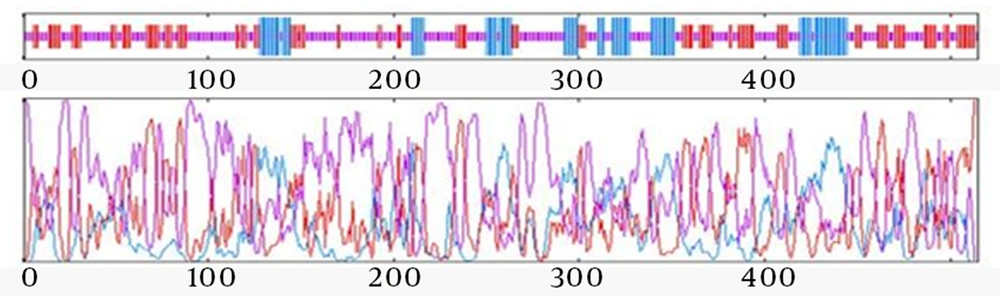
Figure 2 illustrates the pattern of secondary structure of ROR1-SEB predicted by GOR IV. Based on our findings, the structural content of ROR1-SEB was composed of 18.64% alpha helix, 27.38% extended strands and 53.98% random coil. Full length of ROR1-SEB is made up of 33 random coils, 30 extended strands and 11 alpha helices. As summarized in Table 1, the secondary structure pattern of the chimeric protein is similar to the extracellular part of ROR1 and SEB.
| Protein | Extended Strand | Alpha Helix | Random Coil |
|---|---|---|---|
| 26.78 | 9.62 | 63.60 | |
| 28.95 | 25.19 | 45.86 | |
| 27.38 | 18.64 | 53.98 |
[object Object]
4.4. Prediction of 3D Structure
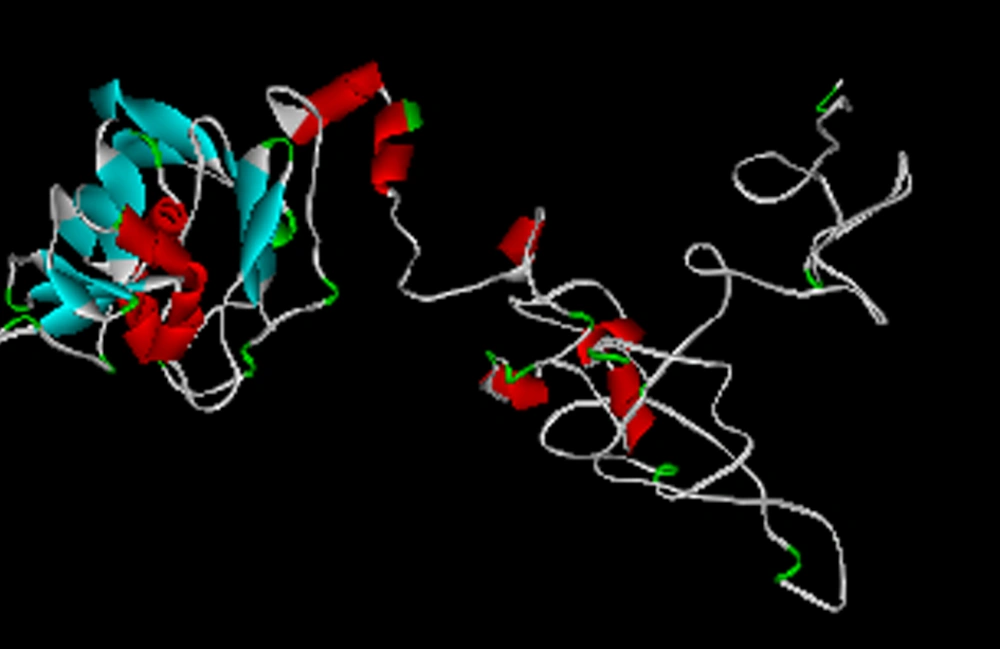
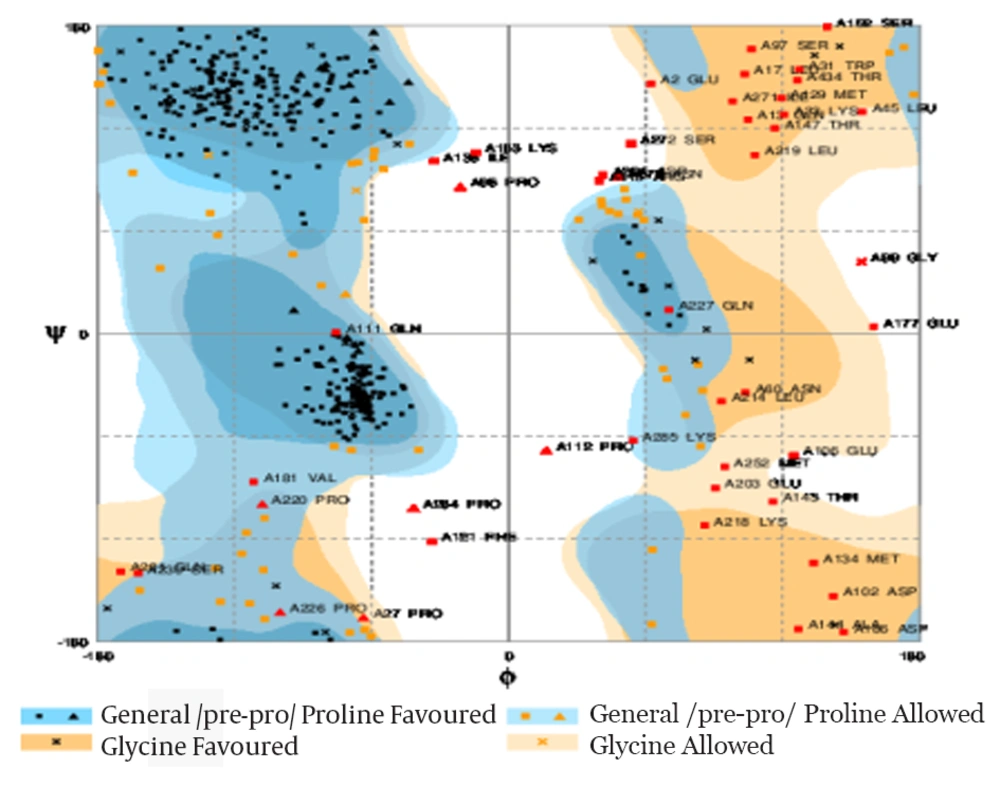
As findings from I-TASSER server, five three-dimensional models were afforded for our designed protein fusing with GSGGSGGSGGSG as a linker. Figure 3 illustrates the best tertiary model predicted for describing protein, which has three separate parts and two domains. The confidence score, as a factor estimates the quality of suggested model was -2.22. What’s more, the expected TM- score and RMSD were 10.9 ± 4.6 Å and 0.53 ± 0.15, respectively. Additionally, we fused two proteins using (HDPVRVS) 2 as an alternative construct. I-TASSER results from predicting tertiary models for this alternative construct showed no appropriate structure therefore we exerted all our analysis only on the first construct. The Ramachandran plot assessment viewed that 80.2% (413 amino acids), 11.1% (57 amino acids) and 8.7% (45 amino acids) were situated in the favored region, allowed region and outlier region, respectively (Figure 4). The quality assessment of the Ramachandran plot revealed that more than 90% of residues located on acceptable (favored and allowed) regions. In accordance with Pdb Viewer analysis, the energy minimization amount was -9536.065 Kcal/mol that portended the plausible stability for our designed construct.
4.5. B-Cell Epitopes
Table 2 listed the epitopes predicted by BCpreds and AAPpreds within the full-length of designing protein. The appropriate epitopes were selected according to cutoff values of 0.8, 0.8 and 0.4 for BCpreds, AAPpreds and VaxiJen, respectively. Moreover, the conformational B cell epitopes were evaluated by two servers termed Discotope and CBTOPE and their data summarized in Tables 3 and 4, respectively. On the other hand, the predicted B cell epitopes were determined in accordance with different parameters including hydrophilicity, flexibility, accessibility, exposed surface, polarity and antigenic propensity with the respective thresholds of 1.9, 2, 1.9, 2.4, 2.3, 1.8 and 1.9, respectively. As demonstrated in Table 5, although applied linker has been determined as a hydrophobe and flexible epitope, it showed no surface exposed epitope, having to interact with antibodies. Table 6 summarizes the predicted epitopes, which can simultaneously interact with B cell, MHC class I and class II with the highest number.
| Position | BCpred Epitope | Score | VaxiJen | Position | AAPpred Epitope | Score | VaxiJen |
|---|---|---|---|---|---|---|---|
| KFGPPPTASPGYSDEYEEDG | 1 | 0.1212 | 279 | ESQPDPKPDELHKSSKFTGL | 1 | 0.9262 | |
| GSGGSGGSGGSGMYKRLFIS | 1 | 0.8888 | 34 | NDAPVVQEPRRLSFRSTIYG | 1 | 1.0983 | |
| NKMVDSKDVKIEVYLTTKKK | 1 | 0.7183 | 494 | MYNDNKMVDSKDVKIEVYLT | 1 | 0.3562 | |
| GNPPPTIRWFKNDAPVVQEP | 0.999 | 0.8779 | 88 | VKFGPPPTASPGYSDEYEED | 1 | 0.0531 | |
| AESQPDPKPDELHKSSKFTG | 0.968 | 0.7827 | 468 | IENENSFWYDMMPAPGDKFD | 1 | 0.6351 | |
| RLKLPNCEDLPQPESPEAAN | 0.915 | 0.7060 | 226 | PQPESPEAANCIRIGSGGSG | 1 | 1.1506 | |
| DEPMNNITTSLGQTAELHCK | 0.881 | 0.8571 | 12 | GQTAELHCKVSGNPPPTIRW | 1 | 1.0859 | |
| NENSFWYDMMPAPGDKFDQS | 0.868 | 0.5261 | 392 | MYGGVTEHNGNQLDKYRSIT | 1 | 1.4149 | |
| LCHYAFPYCDETSSVPKPRD | 0.867 | 0.6159 | 351 | LADKYKDKYVDVFGANYYYQ | 0.869 | 0.3303 | |
| DKYKDKYVDVFGANYYYQCY | 0.805 | 0.3624 | 62 | DTTDTGYFQCVATNGKEVVS | 0.092 | 0.6347 | |
| SKKTNDINSHQTDKRKTCMY | 0.788 | 0.2889 | |||||
| TTDTGYFQCVATNGKEVVSS | 0.779 | 0.6283 | |||||
| IKDTKLGNYDNVRVEFKNKD | 0.74 | 1.0925 |
| Amino Acid | Position | Contact Number | Discotop Score |
|---|---|---|---|
| 1- 6 | 1,16, 7, 19, 14, 6 | 0.515, -0.842, 0.634, -1.893, -1.431, -1.81 | |
| 8-14 | 9,15, 4, 0,2, 8, 3 | -2.375, -3.332, -1.213, -0.741, -0.858, -2.744, -2.486 | |
| 16-31 | 12, 22, 14, 21, 6, 20, 2, 14, 2, 4, 0, 8, 10, 16, 16, 15 | -3.248, -3.584, -2.197, -2.664, 0.005, -1.423, 1.683, 1.138, 2.601, 2.668, 3.306, 2.322, 1.075, -0.466, -1.82, -3.433 | |
| 41-46 | 3, 0, 2, 9, 16, 2 | -3.138, -1.218, -0.279, -0.751, -2.35, -0.849 | |
| 48-50 | 2, 15, 1 | -1.325, -3.416, -2.109 | |
| 53-54 | 5, 2 | -2.365, -3.229 | |
| 65-66 | 0, 3 | -2.461, -2.745 | |
| 91-92 | 11, 3 | -3.196, -2.418 | |
| 94-95 | 12, 3 | -2.906, -2.069 | |
| 97-98 | 0, 3 | -0.66, -2.636 | |
| 227-231 | 3, 10. 3, 7, 1 | -2.599, -2.678, -2.72, -1.993, -2.921 | |
| 240-252 | 7, 13, 8, 11, 3, 9, 10, 4, 3, 5, 6, 8, 12 | -0.897, -1.514, 0.086, 1.325, 3.232, 1.298, 0.689, 1.171, 0.916, -0.147, -1.799, -3.045, -3.294 | |
| 281-301 | 18, 12. 9, 21, 5, 8, 20, 7, 26, 26, 9, 28, 8, 12, 31, 2, 15, 7, 23, 4, 23 | -3.327, -2.155, -1.736, 0.317, 4.474, 3.591, 3.562, 5.622, 1.649, 2.357, 5.92, 2.29, 5.097, 4.013, 1.748, 4.057, 2.026, 2.515, 0.336, 1.913, -1.357 | |
| 303-304 | 20, 13 | -3.060, -2.894 | |
| 307-308 | 13, 6 | -3.677, -3.135 | |
| 314-322 | 5, 6, 24, 6, 34, 25, 21, 16, 21 | 0.162, 0.84, -2.32, 1.272, -2.386, -2.22, -1.957, -1.63, -3.409 | |
| 324 | 5 | -2.481 | |
| 328 | 18 | -2.524 | |
| 330 | 13 | -0.9 | |
| 332-341 | 4, 24, 3, 7, 14, 2, 14, 23, 10, 24 | -0.679, -3.185, -0.899, -1.087, -1.69, 0.423, -1.37, -2.883, -1.184, -2.874 | |
| 348-350 | 6, 1, 3 | -2.495, -1.55, 0.181 | |
| 352-354 | 20, 3, 17 | -3.396, -0.961, -1.437 | |
| 356-361 | 15, 2, 21, 7, 31, 18 | -1.009, 2.356, -0.582, 0.301, -3.061, -2.35 | |
| 369-370 | 14, 13 | -2.388, -2.661 | |
| 372 | 1 | -1.604 | |
| 374-389 | 5, 23, 14, 17, 4, 0, 11, 2, 0, 2, 14, 2, 3, 27, 13, 24 | -0.966, -2.147, 0.302, 0.523, 2, 4.992, 2.571, 5.378, 5.726, 5.674, 1.902, 3.401, 3.032, -1.421, -1.065, -3.076 | |
| 397-409 | 30, 10, 18, 6, 4, 25, 10, 16, 0, 5, 10, 23, 7 | -3.34, 0.052, 0.2, 2.671, 2.901, -0.518, 1.283, 0.41, 2.285, 0.737, -0.385, -2.808, -2.969 | |
| 416-419 | 15, 7, 1, 4 | -2.338, 1.239, -0.095, -3.019 | |
| 427 | 8 | -3.343 | |
| 429-432 | 14, 26, 9, 16 | 0.718, -1.554, 1.49, 1.2 | |
| 449 | 20 | -3.366 | |
| 451 | 13 | -1.034 | |
| 453-464 | 27, 13, 0, 2, 9, 38, 10, 29, 12, 16, 31, 23 | -1.439, 0.886, 4.002, 4.59, 2.755, -0.539, 2.565, -0.529, 3.315, 1.136, -1.455, -1.643 | |
| 476-477 | 28, 21 | -3.446, 1.018 | |
| 479-487 | 30, 30, 10, 11, 5, 0, 6, 29, 10 | 0.017, 1.22, 4.186, 4.109, 3.864, 4.003, 2.213, -0.442, 0.496 | |
| 490 | 9 | -2.104 | |
| 496-497 | 17, 9 | -3.3, -2.01 | |
| 499 | 18 | -2.508 | |
| 502 | 9 | -2.631 | |
| 504 | 8 | -3.009 | |
| 511-517 | 17, 27,16, 8, 12, 8, 8 | -3.5, -3.402, 0.009, 1.758, 3.135, 3.925, 2.506 |
| Amino acid | Position | Probability Scale | Amino Acid | Position | Probability Scale | Amino Acid | Position | Probability Scale |
|---|---|---|---|---|---|---|---|---|
| 1-4 | 4 | M | 129 | 5 | VFGAN | 362-366 | 4 | |
| 18-19 | 4 | ESLH | 130-133 | 4 | YYQCYFSKKTNDI | 368-380 | 4 | |
| 21 | 4 | M | 134 | 5 | S | 382 | 4 | |
| 22 | 6 | QG | 135-136 | 4 | QT | 384-385 | 4 | |
| 23-24 | 5 | NQITAA | 140-145 | 4 | D | 386 | 5 | |
| 25 | 4 | M | 148 | 4 | K | 387 | 7 | |
| 26 | 5 | T | 151 | 4 | R | 388 | 5 | |
| 27 | 4 | S | 152 | 5 | K | 389 | 6 | |
| 28 | 5 | SH | 153-154 | 4 | TC | 390-391 | 5 | |
| 29-34 | 4 | C | 159 | 4 | M | 392 | 4 | |
| 37-46 | 4 | PSLCHYAFPYC | 165-175 | 4 | YGGVTEH | 393-398 | 5 | |
| 51 | 4 | DETS | 176-179 | 5 | NGNQLDKY | 400-407 | 6 | |
| 55-58 | 4 | SVPKPRDLCRDE | 180-191 | 4 | R | 408 | 7 | |
| 60-63 | 4 | C | 192 | 5 | S | 409 | 6 | |
| 66 | 4 | EILENVLCQTEYI | 193-205 | 5 | IT | 410-411 | 5 | |
| 67 | 5 | PESPEAANCI | 228-237 | 4 | V | 412 | 4 | |
| 68-69 | 4 | V | 276 | 4 | G | 418 | 4 | |
| 72-74 | 4 | AESQPDPKPDEL | 278-289 | 4 | SFDV | 423-426 | 4 | |
| 76-77 | 4 | H | 290 | 5 | LV | 446-447 | 4 | |
| 81-83 | 4 | KSS | 291-293 | 4 | K | 448 | 5 | |
| 94-96 | 4 | MENMK | 299-303 | 4 | NKKLYEFN | 449-456 | 4 | |
| 101-109 | 4 | LY | 305-306 | 4 | DQSKYLMMY | 487-495 | 4 | |
| 110 | 5 | D | 308 | 4 | D | 497 | 4 | |
| 111-112 | 4 | H | 310 | 4 | KMVDSKD | 499-505 | 4 | |
| 119-122 | 4 | TKLGNY | 334-339 | 4 | KI | 507-508 | 4 | |
| 125-126 | 4 | NV | 341-342 | 4 | Y | 511 | 4 | |
| 127 | 5 | VEFKN | 344-348 | 4 | ||||
| 128 | 4 | DL | 350-351 | 4 |
| Prediction Parameters | Epitope Position |
|---|---|
| 59 - 68, 97 - 108, 135 - 141, 174 - 182, 226 - 235, 240 - 252, 278 - 288, 307 - 313, 347 - 359, 374 - 391, 397 - 406, 425 - 423, 456 - 463, 483 - 490, 496 - 503 | |
| 153 - 159, 172 - 181, 237 - 250, 277 - 283, 287 - 295, 362 - 370, 372 - 388, 423 - 431, 481 - 487, 499 - 505, 510 - 517 | |
| 1 - 7, 22 - 49, 54 - 68, 89 - 109, 124 - 130, 135 - 141, 177 - 190, 217 - 235, 278 - 297, 303 - 312, 329 - 361, 363 - 392, 396 - 410, 412 - 418, 423 - 494, 500 - 517 | |
| 40 - 46, 101 - 107, 179 - 185, 279 - 294, 329 - 335, 342 - 361, 372 - 391, 401 - 409, 425 - 437, 445 - 456, 484 - 491, 499 - 505, 511 - 517 | |
| 14 - 22, 38 - 49, 53 - 61, 100 - 109, 133 - 139, 182 - 196, 254 - 266, 282 - 296, 340 - 361, 382 - 393, 413 - 419, 426 - 437, 442 - 456, 465 - 472, 484 - 490, 499 - 517 | |
| 16 - 23, 37 - 43, 66 - 72, 74 - 92, 164 - 170, 191 - 204, 255 - 265, 267 - 276, 319 - 331, 357 - 364, 366 - 375, 409 - 416, 427 - 430, 440 - 448, 505 - 514 |
| Sequence | Number of Mhc Class I Binding Alleles | Number of MHC Class II Binding Alleles | Vaxijen Scores | Total Number of MHC Binding Alleles |
|---|---|---|---|---|
| 3 | 30 | 0.5520 | 33 | |
| 14 | 26 | -0.2150 | 40 | |
| 4 | 5 | 0.6692 | 9 | |
| 14 | 0 | 0.6486 | 14 | |
| 5 | 6 | -0.2295 | 11 |
4.6. Allergenicity Property
Based on outcome from AlgPred and SDAP database, our construct viewed no allergenic sites along its sequence. Furthermore, it had no great similarity to the allergen listed in SDAP library.
4.7. Protein Solubility Prediction
According to outputs from recombinant protein solubility prediction, our designed construct possesses 36.6% a solubility chance after overexpressin in E.coli.
5. Discussion
Development of efficacious therapeutic strategy for some of the resistant malignancies is the main emergency of health organization all around the world. Nowadays, design of appropriate and safe vaccines which stimulate the immune response actively or passively, are the hot topic in the field of reverse vaccinology. This area is closely related to computational vaccinology that recruits discrepant informatics tools to predict efficient T- and B- cell functional epitopes to improve the properties of an antigen based vaccine (24, 25).
The principal purpose of the current study was to design a unique construct, including two antigenic parts, which adjoined together through hydrophobic linker (26, 27). Theoretically, our structural model could augment immunogenicity of ROR-1 protein and owing to the presence of staphylococcal enterotoxin B as a potent superantigen; its probability evokes a wide cellular or humoral anti-tumor immune response. As respects to the momentous role of linker in representing the pattern of various epitopes throughout the chimeric protein besides maintenance of its functional properties, the linker selection is a key point in designing of the fusion protein (26). In this study a flexible linker, GSGGSGGSGGSG With 12aa, was used to separate domains of two proteins. Aria et al. repotted the multimerizing property of short helical linker compared with longer ones. Furthermore, a flexible linker based on shorter conformation plays an efficient role in comparison to those with the helical linker (26). To predict the secondary structure of a chimeric protein, the GOR method was applied. This software allows estimating the possible secondary structure of each amino acid together with its impact on the condition and structure of adjacent amino acids. The most abundant structure within our fusion protein was a random coil that could be due to the presence of a high amount of hydrophobic amino acids such as glycin. In accordance with finding from the physico-chemical parameter analysis, our fusion protein had an acidic nature with the high extinction coefficient at 280 nm, which is owing to high content of Cys, Trp and Tyr. In contrast to partial instability of our fusion protein, its estimated high alphabetic index infers to protein stability in a broad range of temperature.
One of the most important problems in the designing of recombinant protein is the biologic functional characteristics. Although prediction of secondary structure by ab-initio methods or folding recognition is able to detect some of the limitations (28), prediction of three- dimensional structure through comparative and ab-initio methods attenuates several errors (29, 30). The three-dimensional model of the fusion protein ROR-1-SEB protein was accounted using the I-TASSER server (14) according to their confidence score (C-score), Z-score, RMSD and TM-score. This server suggests five models for our chimeric sequence that model 2 had more c-score between them, therefore it selected for further evaluation. Expected TM-score obtained 0.53 ± 0.15, which accredited the validity of the model. A TM-score more than 0.5 portend accuracy of topology. Data from Procheck Ramachandran plot demonstrated the stability of the fusion protein. Thereabout, 8.7% of the residues located in outline region, which, could presumably be owing to fusion. Since the purpose of designing a vaccine is the generation and selection of a candidate with the potential stimulation of strong responses (31), we analyzed the epitope maps for B cell and T cells. At first, we predicted linear B-cell epitope overall the chimeric sequence using BCpreds. Moreover, for recognizing the epitopes involved in antibody-antigen interaction, the estimation of conformational epitopes is an essential in the computational vaccine design which executed using both structure and sequence information based method, including DiscoTope and CBTOPE, respectively. Our result showed the copious b-cell epitopes, though some of the solely predicted by one method. In order to predict the map of T-cell epitope and binding affinity to both classes of MHC molecules, Propred and Propred-1 were applied. Numorous T-cell epitope with a high antigenicity score were suggested by two methods, but we only selected those epitope that is simultaneously proposing as B-cell and T-cell epitope (Table 6). Our result suggested that our structural model represented the epitope that are capable to be a stimulant for both T-cell and B- cell mediated immune responses. At last, this structure showed no significant resemblance with the allergen in the SDAP library.