1. Introduction
Evans and Batsakis first described PLGA as a distinct entity in 1984 as it is characterized as a tumor with low grade biologic behavior and diverse architectural pattern (1). Previously lobular carcinoma or terminal duct carcinoma of minor salivary glands were the terms used to describe the histological appearance of this tumor (2, 3). PLGA is an indolent tumor, exclusively arising in the minor salivary glands (4, 5). Waldron et al. has reported that it is the third most common intra oral malignancy occurring in minor salivary glands with an incidence rate of 26.4% among intraoral malignancies (6). Though it is common in minor salivary glands, two cases have reported in parotid gland (7) and one in the sublingual gland (8). As the tumor is asymptomatic, many times it is only diagnosed during routine diagnostic evaluation (9).
2. Case Presentation
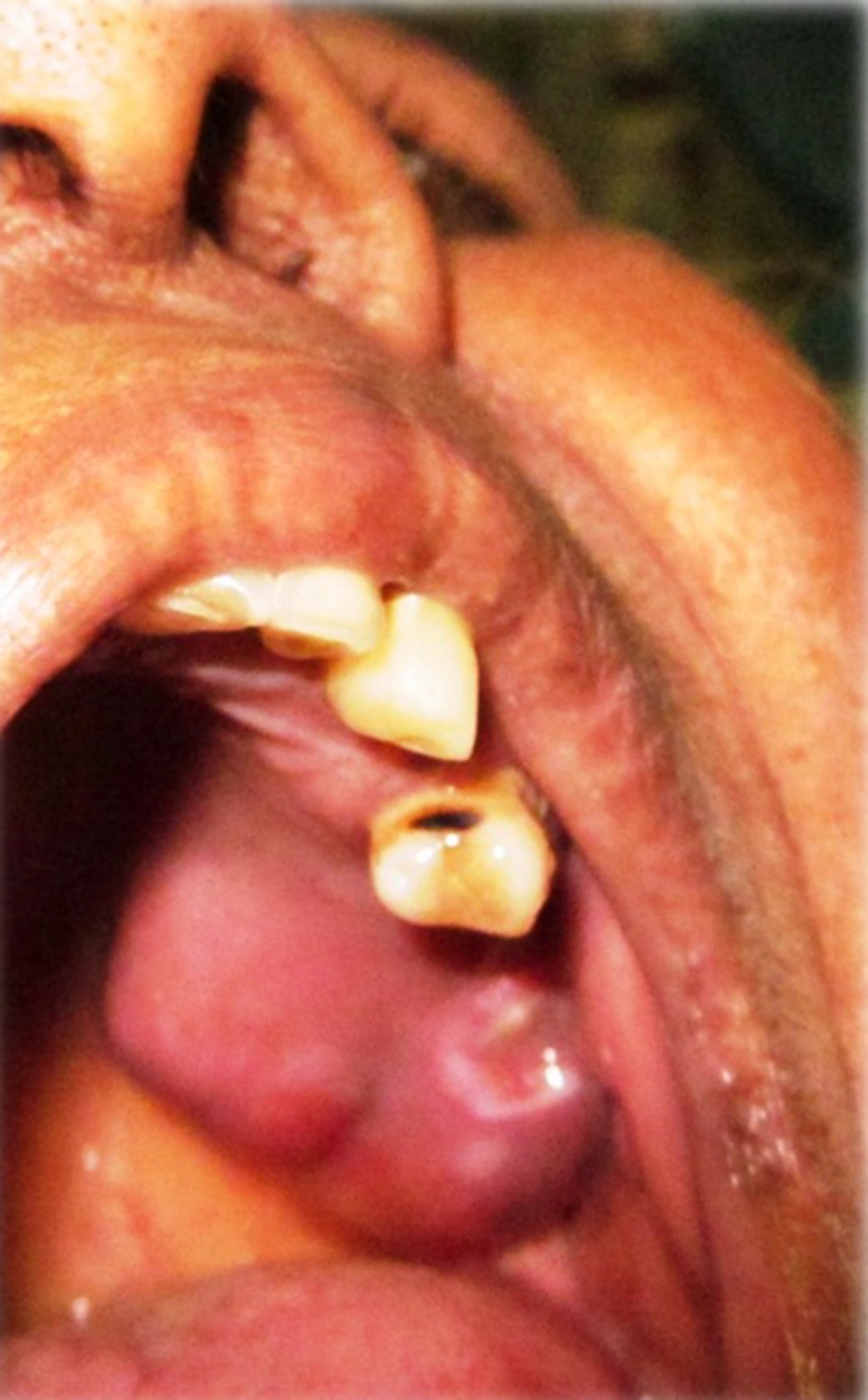
A 63-year-old patient had reported at our institute with the chief complaint of swelling in relation to her upper left back tooth region for 15 days which has associated with pain for 3 days. The swelling had been gradual in onset and has slowly increased in size. There was no history of any secondary changes and the pain associated with the swelling was of a severe, throbbing type in relation to the middle third of face, which was aggravated on chewing food, causing difficulty in food intake. There has been no history of nasal stuffiness or paresthesia. Her general examination has shown that review of her systems were normal except for bilateral palpable submandibular lymph nodes of size 1 × 2 cm which were soft, tender and mobile with no fixation to the underlying structures. Intraorally, on inspection, a solitary diffuse swelling of size 3 × 2 cm was present in relation to the left posterior part of the hard palate (Figure 1). The swelling has extended anteriorly up to the 1st molar and posteriorly to the soft palate. It has extended medially from the midline to the buccal vestibule laterally. The surface of the lesion appeared smooth with necrotic slough seen in the buccal vestibule. On palpation, all the inspectory findings in relation to the site, size and extent of the lesion have confirmed. The swelling was tender on palpation and firm in consistency. There was also a root stump in relation to the left second molar which was tender on percussion. Aspiration of the lesion was positive and yielded blood tinged pus. The provisional diagnosis was given as a minor salivary gland tumor with 2 other differential diagnosis namely palatal abscess and consolidated abscess in relation to left second molar.
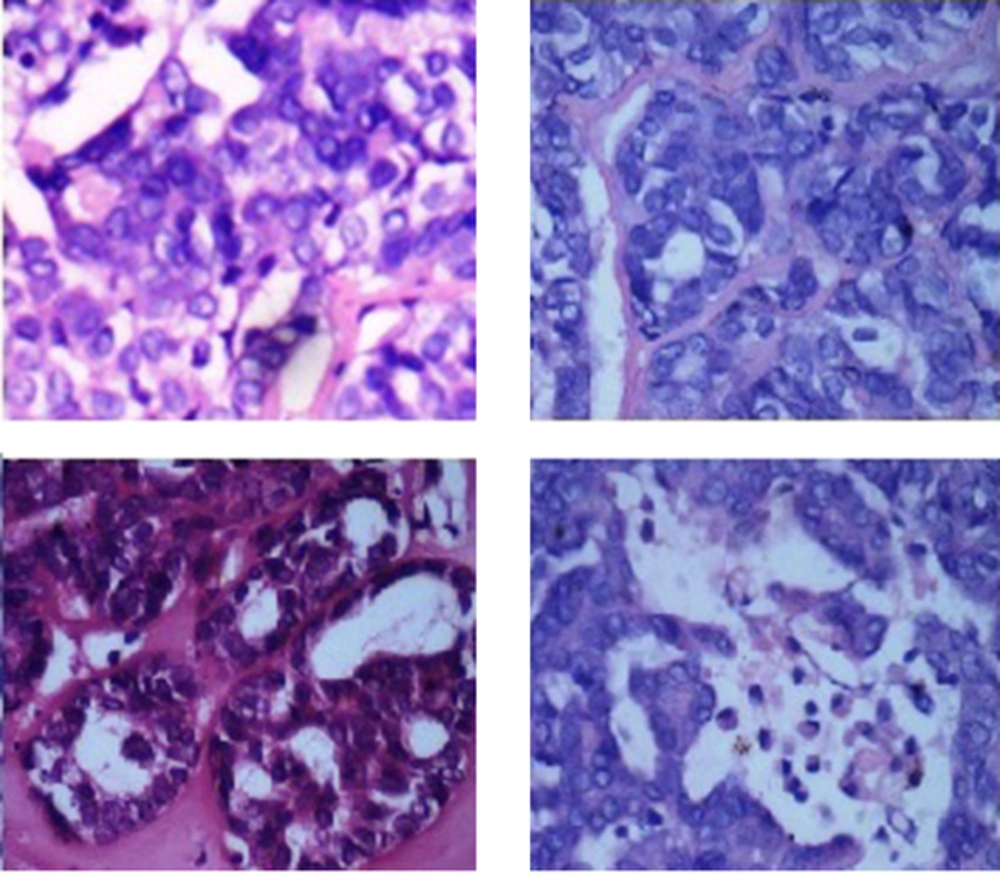
Routine hematologic investigations were normal and the radiologic investigations included a maxillary occlusal radiograph which had revealed an ill-defined radiolucency of size 1 × 2 cm present in relation to left third molar with ill-defined borders and an OPG has revealed a root stump in relation to left third molar, there was no evidence of erosion or invasion of the underlying structures. The CT scan revealed involvement of the posterior aspect of the left hard palate, the alveolar process and buccal vestibule and multiple bilateral lymph nodes with possibility of pathology. An incisional biopsy of the lesion has done, and the histopathological features were indicative of uniform tumor cells exhibiting moderate eosinophilic to amphophilic cytoplasm, vesicular nuclei and occasional prominent nucleoli. Cytologically bland tumor cells have shown various patterns like nest, ductular, tubular and papillary patterns (Figure 2) showing apoptotic bodies. The investing stroma ranged from fibrous to hyalinised. The features were indicative of a diagnosis of polymorphous low grade adenocarcinoma. A fine needle aspiration biopsy of the left submandibular lymph node was negative.
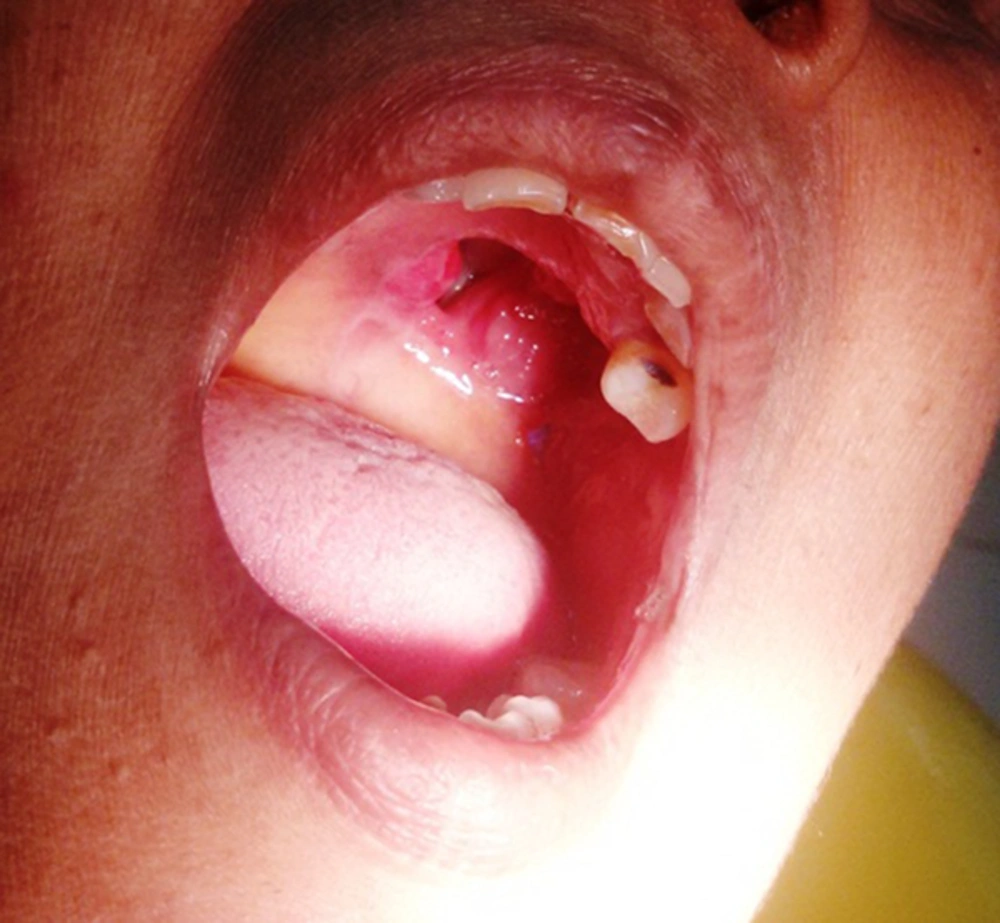
A treatment plan of surgical excision of the primary lesion with immediate obturator placement was made. Accordingly the patient was administered general anesthesia and through an intraoral approach, palatal and buccal mucoperiosteal flaps were elevated and the lesion has exposed. Partial maxillectomy was done maintaining a tumor free margin of 2 cm and including the involved alveolar process. The sinonasal defect was packed with a ribbon guaze soaked in antibiotic ointment. Rehabilitation with immediate obturator placement was done, and the patient extubated uneventfully. The specimen was sent for histopathological examination. Regular follow up was done postoperatively, initially every day to change the gauze packing and later review was done every month. The wound healing was satisfactory showing healthy granulation tissue at the periphery of the lesion (Figure 3). Since there were no signs of recurrence, after 6 months of follow up, the patient was given a definitive functional obturator. The patient is at present under regular follow up.
3. Discussion
PLGA is a distinct entity due to its architectural diversity, cytological uniformity, and indolent clinical behavior (2, 10). Its clinical behavior is characterized by slow rate of growth, absence of symptoms, less aggressiveness, minimal metastatic potential and good prognosis (11). The lesion described in this patient was similar to the ones previously described in literature in terms of the location, indolent course, minimal symptoms, delayed detection and histopathological features.
From the past literature it is seen that PLGA is more frequent in females than in males with the female to male ratio being 3:1 (12). Also adults from the age group of the 3 rd and 7 th decades were more frequently affected with the peak prevalence being in the 5 th and 6 th decades. In accordance with the prevalence of PLGA in literature our patient was also a female in her 6 th decade.
Site of predilection is more in favor of the palate (49 - 77.8%) followed by either the upper lip or buccal mucosa (7.4 - 13.4%) and rarely could involve floor of mouth, lower lip, alveolar ridge and tongue (1, 13-15). Literature has shown that PLGA could also arise in lung (16) maxilla (17), parotid gland (18) and submandibular gland (19). In our case the tumor had presented as an asymptomatic swelling with smooth margins and surface and was seen in the soft palate extending anteriorly to the hard palate.
There were sporadic reports of metastasis sometimes even transformation to a high grade adenocarcinoma, sometimes ending in mortality as reported by Evans et al. (1). Cervical lymph node metastasis was rare with reported incidence of 5 - 15% and was more commonly seen in recurrent tumor than the initial disease (17). Extra palatal PLGAs had presented with significant papillary growth or arising from the ventral surface of the tongue and frequently metastasize to cervical lymph nodes (18). Distant metastasis was very rare with an incidence of 7.5% and the site involved is the lung which has attributed to the inadequate control of the disease (19). In our case neck and chest imaging was done and there was no evidence of distant metastasis. Many times a small incisional biopsy specimen was sufficient for diagnosis if the lesion was small or multiple incisional biopsy samples might be necessary for large tumors especially in case the differential diagnosis includes a salivary gland malignancy (1, 20). The tumor margins should also be examined to assess the presence of infiltration (1, 16, 17). In our patient, postoperative histopathological analysis of the excised specimen had indicated a PLGA which had bone invasion.
3.1. Treatment
Wide local excision should be the ideal treatment of choice. If positive or close surgical margins is there, post-operative radiotherapy is recommended but it has not shown to alter outcome in patients without neck node metastasis. Adjuvant radiotherapy is indicated if there is cervical lymph node metastasis. The recurrence rate for PLGA is minimal following wide excision and if present Radical excision is warranted (12). In our case partial maxillectomy was followed by placement of an obturator.
3.2. Conclusions
In general minor salivary gland tumors are relatively uncommon pathologies. PLGA is said to be the second most common minor salivary gland tumor. It is unique for its architectural diversity, cytological uniformity and subtle clinical behavior. More case reports are needed to understand the site, age, sex, and prevalence and histological typing of this tumor which would help in the proper diagnosis and appropriate management of polymorphous low grade adenocarcinoma.