1. Background
Gastric carcinoma (GC) remains a significant threat among non-communicable diseases, particularly in underdeveloped and developing countries, where an estimated 70% of new cases will have emerged by 2020 (1). Studies have suggested an inverse relationship between cancer incidence and socioeconomic factors for some malignant cancers, including esophageal, breast, and prostate cancers (2). Prior research regarding socioeconomic status (SES) and health indexes identifies SES as one of the most important variables affecting health-related quality of life (3), and suggests its importance as a predictor of disease morbidity and mortality (4), as well as long-term survival for many cancers (5, 6).
In disadvantaged countries, GC is more prevalent among lower socioeconomic classes, perhaps due to difficulties in accessing remote health centers. As a consequence, most cases have reached advanced clinical stages by the point of diagnosis (7, 8). Several corresponding studies cite the stage at diagnosis as a dependent variable for SES although the reasons for this hypothesis are yet unclear (9). Additional studies suggest that late diagnosis and advanced stages of various cancers are more common in less educated individuals (10), while others conclude that low survival rates are linked to the smoking epidemic model and differences in the social dissemination of smoking (11). In Turin, a GC patient’s level of education was a strong predictor of their treatment (12), whereby lower social class was associated with poorer treatment and, consequently, lower rates of survival (13).
Despite evaluation of socioeconomic inequality in health from the past in the world (developed countries in particular) this approach is newly introduced in Iran for a few health outcomes such as child mortality rate (14), mental health (15), risk factors of diabetes and non communicable disease (16). What follows is that socioeconomic inequalities in survival cancer are avoidable, and their reduction can be an achievable goal for coming decades but we have to provide the necessary knowledge in this context. There are different indexes for evaluating inequalities in health. Concentration index (CI) is one common measure to this assessment. Inequalities can be decomposed into its determinants to calculate the share of contributions to inequality. We conducted this cross-sectional study to examine possible associations between socioeconomic inequality and survival risk factors in patients that were diagnosed with GC and this paper provides the decomposed contributions of socio-economic determinants in GC survival.
2. Methods
2.1. Study Population
For the purposes of this study, we used census method data analysis to conduct a cross-sectional study which included eligible patients diagnosed with upper gastric cancers in Kurdistan Province of western Iran. Our sample included 249 patients who had been diagnosed with GC and registered in the pathological sector between 1 January, 2008 and 31 December, 2013. The study excluded cases in which the patient had not received follow-up consultation (10 patients), the diagnosis had involved illegible data (2 patients), or the patient had emigrated or lost contact (2 patients). On the whole, 235 patients with GC were enrolled in the study. We followed all recruited patient and the exact date of death were obtained from official death certificates, maximum duration of follow-up was 90 months. Survival time was characterized as the number of months from final diagnosis until death or latest follow-up, and outcome was defined as death due to GC at any time during the study period, or survival by the end of the follow-up period.
2.2. Socioeconomic Database
Information regarding the relationship between socioeconomic status and GC survival risk factors was retrieved from three sources: databases, family medical history, and telephone interviews. We first assessed medical records using the hospital database, which integrates existing demographic data using characteristics such as age, sex, and residence (urban or rural), as well as histological records such as stage of tumor at diagnosis. The second step involved extracting medical records to collect data on past medical history of gastrointestinal diseases and family history of gastric cancer in first-degree relatives. Finally, we conducted telephone interviews with the nearest relative of the respective patient, and asked targeted questions to ascertain their socioeconomic status. Questions included personal habits such as smoking, as well as pertinent data such employment history, education level, and economic status (determined by household assets).
2.3. Analysis
In the present study, SES was calculated using polychoric correlation matrix procedure in order to identify variables with greater impact on the whole variance. Using this procedure, new variables that represent SES were identified (17). Initially, we created dummy (0/1) for nominal variables such as residence and job status, and a total of 8 variables were inserted in the polychoric correlation matrix.
Inequality is calculated by measuring the concentration index (CI). According to the Wag staff article, in 1991 (18), most researchers have used this index to measure inequality. The CI is the cumulative percentage of variables against the cumulative percentage of population, ranked by economic index from the poorest to the richest. If there is no inequality in the distribution, CI is zero. When the curve is above of equality line CI is negative and variable is concentrated in those with low SES and positive CI represents more concentration of variable in those with high-grade SES. By the mathematical formula, CI can be computed as twice the covariance of the health variable and a person’s rank in terms of economic status, divided by the mean of the health variable C = 2/μ cov(YiRi). Where yi and Ri are respectively the health status of the ith individual and the fractional rank of the ith individual (in terms of the index of household economic status); μ is the mean of the health and cov denotes the covariance (19).
Socioeconomic inequality decomposition approach proposed by Wagstaff et al. (20) is used to determine contribution of socioeconomic covariates to inequality. This approach allows one to determine the rate of participation variables to inequality. We used the decomposition approach for decomposing socioeconomic determinants analysis, quintiles 1 and 2 and quintiles 3, 4, and 5 were grouped together. This created a binary low SES variable including socioeconomic in the bottom 40% of this approach allows one to determine the rate of variables’ contribution to inequality. Afterwards, binary variable illiterate and primary school were grouped for decomposition analysis. For this approach, we denote total score of SES by y and the set of covariates by X = x1, x2, …, xk linear model using an approach, y α + ∑ βkXk + ∈ were, α and ∈ denote respectively, constant and error term. In the next step, we managed to obtain the contribution of each determinant to inequality by multiplying the elasticity of each determinant by its concentration index (βkXk/μ)Ck where μ is the mean of y and CIs for determinants (Ck). This is the absolute contribution of each determinant to the measured inequality. In the final step, we calculate contribution percentage of each determinant simply through dividing its absolute contribution by the concentration index of the health variable (βkXk/μ)Ck /C (21).
In this study, death is considered as a binary outcome. Thus, to obtain the coefficients, a logistic regression was applied. The appropriate regression model for the linear decomposition in our study method will be: Ln odds (death) = α + ∑βixi + εi CI of the outcome variable based on the Logit model showing the degree of inequality in the natural logarithm of the predicted odds of death.
In this study we used the standard statistical software, STATA (version 11.2).
3. Results
Table 1 shows the characteristics of patients with GC. 58.5 % of the study sample entailed patients above 65 years; of all participants, 74.5% were male; the majority of them allotted to illiterate subgroup (41%); stages 2-3 (59.5%); middle household economic status (54.4%); not having medical history of gastrointestinal diseases (64.1%) and having no family history of GC in first-degree relatives (48.1)%. The adjusted associations between GC death and its determinants are shown in Table 2. We can see that, as expected, increasing age shows protective effects on GC deaths.
| Determinants | Patientsa |
|---|---|
| Age group, y | |
| < 45 | 9 (3.8) |
| 45 to 65 | 90 (37.7) |
| > 65 | 139 (58.8) |
| Sex | |
| Male | 178 (74.5) |
| Female | 61 (25.5) |
| Residence | |
| City | 120 (50.2) |
| Village | 119 (49.8) |
| Job | |
| Housewife | 52 (21.8) |
| Farmer | 53 (22.1) |
| Employee | 14 (5.9) |
| Free Job | 70 (29.3) |
| Unknown | 50 (21.0) |
| Educational level | |
| Illiterate | 98 (41.0) |
| Primary school | 79 (33.1) |
| Guidance/high school | 14 (5.9) |
| University | 4 (1.7) |
| Unknown | 44 (18.4) |
| Household economic status | |
| Low | 87 (36.4) |
| Middle | 130 (54.4) |
| Well | 22 (9.2) |
| Stage of disease | |
| Stages 2 - 3 | 142 (59.5) |
| Stage 1 | 25 (10.4) |
| Unknown | 72 (30.1) |
| Past medical history of gastrointestinal diseases | |
| Yes | 49 (20.5) |
| No | 153 (64.1) |
| Unknown | 37 (15.4) |
| Family history of gastric cancer in first-degree relatives | |
| Yes | 87 (36.4) |
| No | 115 (48.1) |
| Unknown | 37 (15.4) |
| Smoking status | |
| Yes | 103 (43.2) |
| No | 99 (41.4) |
| Unknown | 37 (15.4) |
Summary Statistics of the Gastric Cancer Patients and Its Determinants
| Determinants | Coefficient | P Value | Adjusted Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Low | High | ||||
| Age group (1 - 45) | 1 | ||||
| Age group ( 46 - 55) | 0.875 | 0.031 | 1.19 | 0.084 | 3.03 |
| Age group (< 66) | 1.985 | < 0.001 | 2.39 | 1.06 | 6.05 |
| Sex (Female) | 1 | ||||
| Sex (Male) | 0.175 | 0.106 | 1.03 | 0.113 | 15.66 |
| Residence (Urban areas) | 1 | ||||
| Residence (Rural areas) | 0.340 | 0.411 | 1.46 | 0.453 | 4.73 |
| Job (Housewife) | 1 | ||||
| Job (Farmer) | 0.582 | 0.322 | 0.573 | 0.119 | 2.75 |
| Job (employee) | 0.241 | 0.532 | 0.780 | 0.098 | 6.19 |
| Job (Free) | 0.303 | 0.099 | 1.45 | 0.455 | 4.73 |
| Educational level (Illiterate) | 1 | ||||
| Educational level (primary school) | -0.415 | 0.241 | 0.660 | 0.282 | 1.54 |
| Educational level (guidance/high school) | -1.444 | 0.398 | 0.236 | 0.022 | 2.57 |
| Educational level (university) | 1.022 | 0.089 | 2.77 | 0.162 | 8.63 |
| SES ( poorest) | 1 | ||||
| SES (second poorest) | -0.753 | 0.584 | 0.471 | 0.024 | 9.16 |
| SES (middle) | -0.294 | 0.695 | 0.745 | 0.034 | 16.17 |
| SES (second rich) | -0.463 | 0.574 | 0.629 | 0.017 | 17.67 |
| SES ( richest ) | -0.291 | 0.148 | 1.33 | 0.034 | 19.31 |
Adjusted Associations Between Death from GC and Its Determinants Based on the Logit Model
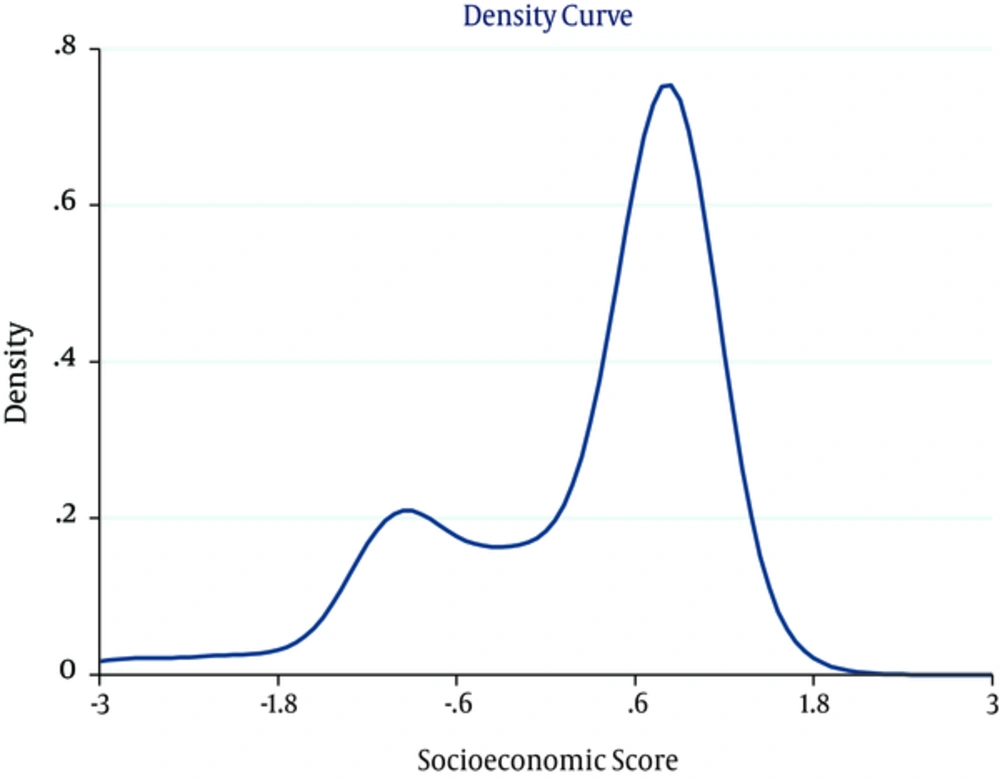
Results of the polychoric correlation matrix shows age, household economic status and educational level had a bigger impact on the whole variance. Overall, 72.3% of whole variance were those of these variables. Asset index variable was denoted by 7 variables and new variable divided to quintiles (Poorest, Second, Middle, Fourth and Richest) (Figure 1).
3.1. Inequality in Survival Determinants of GC
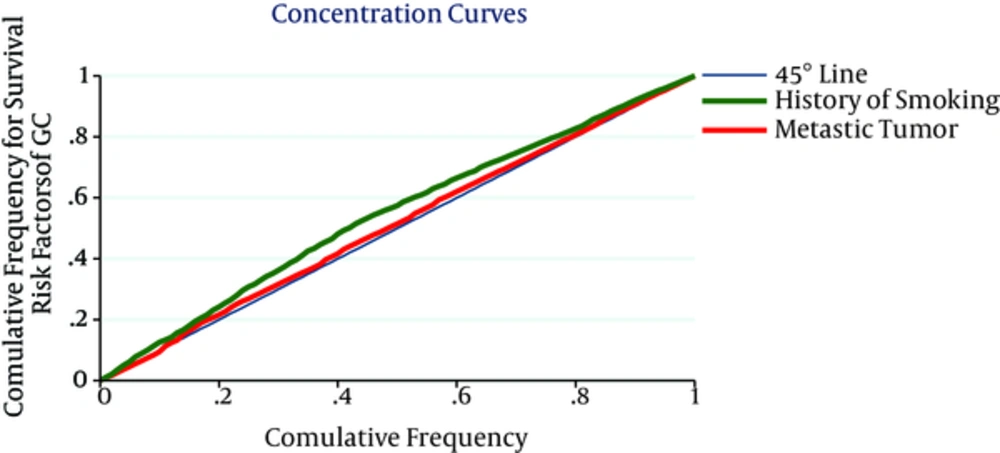
Slope index of CI is shown in Figure 2. The figure displays a patient with lower SES having more risk factors of GC including late diagnosis, having a past medical history of gastrointestinal diseases and history of smoking. The overall CI for late diagnosis, having a past medical history of gastrointestinal diseases, having a family history of gastric cancer in first-degree relatives and positive history of smoking were -0.020 (95% CI = -0.041 - 0.004), -0.106 (95% CI = -0.125 to -0.087), -0.016 (95% CI = -0.035 - 0.010), -0.105 (95% CI = -0.110 to -0.076), respectively. Interoperations of CI assert positive history of smoking is more associated to poorest SES (Table 3).
| Determinants | Concentration Index | STE | 95% Confidence Interval | |
|---|---|---|---|---|
| Low | High | |||
| Stage of disease (2 stage onwards) | -0.020 | 0.012 | -0.041 | 0.004 |
| Having a past medical history of gastrointestinal diseases | -0.106 | 0.012 | -0.125 | -0.084 |
| Having a family history of gastric cancer in first-degree relatives | -0.016 | 0.007 | -0.037 | 0.010 |
| History of smoking | -0.105 | 0.007 | -0.114 | -0.074 |
Concentration Index of Survival Risk Factors by Ranking Variable SES
3.2. Decomposing Socioeconomic Determinants in GC Survival
To do this calculation, we created dummy variable for all variables with several categories and following that inserted them into the model. Table 4 shows that more contribution to socioeconomic inequality in survival of GC because of having a past medical history of gastrointestinal diseases (29%) and history of smoking (18%). Results showed only 49% was not explained by data inserted into the model called residual component.
| Determinants | Coef. | Elasticitya | CI | Absolute Contribution | Relative Contribution, % |
|---|---|---|---|---|---|
| Stage of disease (2 stages onwards) | -0.2834 | 0.003 | -0.019 | 0.003 | 0.022 |
| Having a past medical history of gastrointestinal diseases | -0.8460 | 0.008 | -0.104 | 0.047 | 0.299 |
| Having a family history of gastric cancer in first-degree relatives | 0.0132 | 0.002 | -0.013 | 0.000 | 0.003 |
| History of smoking | -0.6842 | 0.004 | -0.094 | 0.029 | 0.183 |
| Residual | 0.077 | 0.490 |
Decomposition Analysis of Socio-Economic Determinants of Gastric Carcinoma
4. Discussion
Previous research regarding the relationship between GC and SES in developing and developed countries found that instances of GC are more common in people of lower SES. Socioeconomic determinants provide possible explanations for the inequality in GC patient survival.
The current paper reveals the influence of socioeconomic determinants on GC survival risk factors, contributing to previous studies identifying age, race, and socioeconomic factors as significant predictors of survival outcomes for GC patients. In 2003, Newnham reported a significant association between five-year survival rate and deprivation for women with GC, but not for men with GC in either sex (22). Likewise, Whynes (2003) revealed that females in the least-deprived SES tended to live an average of 1.1 years longer than females in the most-deprived SES (23).
As previous studies, the present study investigated the relationship between social differences and the stage of disease at diagnosis, and its impact on survival (24, 25). Results indicate that risk factors such as advanced age, low economic status, regular smoking, past medical history of gastrointestinal diseases, and late diagnosis are more prevalent among people of lower SES. In fact, the present study found that a past medical history of gastrointestinal disease accounted for the majority (29%) of existing socioeconomic inequality in GC survival. These findings are consistent with previous studies focusing on breast carcinoma (26) and colorectal cancer (23) which found that the stage of diagnosis varies between populations and has vast implications for cancer survival (22).
In previous reports, lifestyle and smoking have been identified as significant risk factors for many different cancers (27). Several studies suggest that smoking is more prevalent among lower social classes (28); therefore, the habit has implications for the impact of socioeconomic inequality on survival. Our results support these findings, confirming that smoking is more commonin patients from lower SES levels and therefore a greater risk to these subsets of the population.
In addition to personal habits, access to optimal treatment is one of the most significant explaining factors for differences in SES survival rates, and family history of illness plays a role, as well. The present study found that a history of gastrointestinal disease is more common in low SES communities, revealing that individuals with a history of smoking and gastrointestinal disease also run a greater risk of GC diagnosis.
The present study took a novel approach to illuminate socioeconomic inequality in GC survival, employing the decomposition method to quantify the contributions of socioeconomic determinants on health indicators. Due to the method employed this study lacked survey data such as income, expenditure, or consumption. We attempted to offset this limitation with the use of telephone interviews with close relatives to determine economic status based on household assets. Additionally, we used the common PCA process to construct an index of SES according to characteristics like economic status, education, age, gender, residence, and employment.
4.1. Conclusions
This study calculated the disparate effects of socioeconomic factors on GC survival. Results revealed that risk factors such as smoking, a past medical history of gastrointestinal disease, and late diagnosis are more prevalent among individuals of lower SES, and contribute to lower GC survival rates. Our analyses and findings are particularly valuable for health intervention strategies aimed at achieving equality in health services and ultimately increasing GC survival.