1. Background
Primary spinal cord tumors represent 4.5% of all CNS neoplasms and the most common tumor type is meningioma (24.4%) (1). Intraspinal meningiomas are usually intradural extramedullary tumors (2). Spinal meningiomas are more common in elderly patients, mean age varying from 56 up to 66 years in different studies (3-6); in Sandalcioglu et al.’s study, the age range was 17 to 88 years in a review of 131 patients (3). Spinal meningiomas are slow growing tumors and therefore, they lead to symptoms only when they reach a considerable size to compress the spinal cord, causing local pain; however, in a significant number of patients, the diagnosis is not confirmed until neurologic deficits or gait disturbances become evident (3). Intraspinal meningiomas are most commonly located in thoracic region, followed by cervical and lumbar areas (7-9). There are only few reports in the literature describing postoperative outcome of spinal meningiomas (10-14). In addition, little is known about the different prognostic factors influencing recovery, especially the influence of histopathologic subtype (11).
2. Objectives
This study is carried out to evaluate the effect of some of these factors specially the effect of histopathologic subtype on the postoperative outcome.
3. Patients and Methods
All patients with spinal meningioma referred to Shohada hospital, Tehran, Iran for surgical resection of spinal meningioma between October 1998 and January 2012 were included in this study. The patients’ records including age, sex, address, history, radiologic data, operative notes, tumor location and pathology reports were registered retrospectively. The results of 8 - 120 months post-operative follow up were then classified according to Frankel classification (Table 1).
| Grade | Description |
|---|---|
| Complete neurological injury, no motor or sensory function clinically detected below the level of the injury | |
| Preserved sensation only, no motor function clinically detected below the level of the injury; sensory function remains below the level of the injury but may include only partial function (sacral sparing qualifies as preserved sensation) | |
| Preserved motor non-functional, some motor function observed below the level of the injury, but is of no practical use to the patient | |
| Preserved motor function, useful motor function below the level of the injury; patient can move lower limbs and walk with or without aid, but does not have a normal gait or strength in all motor groups | |
| Normal motor, no clinically detected abnormality in motor or sensory function with normal sphincter function; abnormal reflexes and subjective sensory abnormalities may be present |
Frankel Classification of Neurologic Deficit
All patients had plain X-rays and CT-scan of spine and only 14 (36%) had MRI for localization of tumor. Three pathologists studied histologic slides independently and diagnoses were made according to WHO 2007 criteria.
Statistical analyses were performed using SPSS software version 11.0. Using t-test and Fisher’s exact test, we compared the results. A P value of less than 0.05 was considered statistically significant.
4. Results
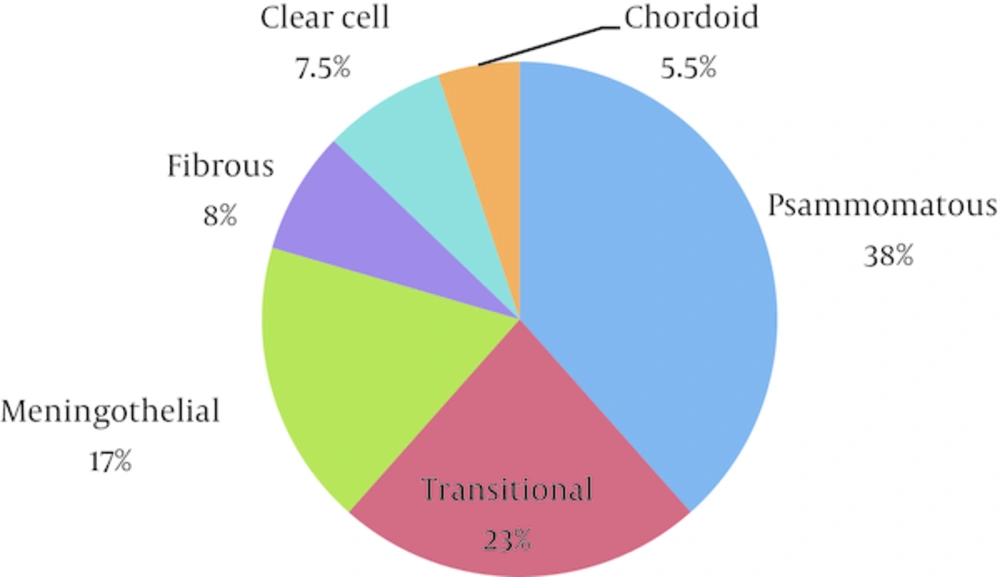
A total number of 39 patients with spinal meningioma were referred to Shohada hospital and underwent neurosurgical resection. Patients’ mean age was 51.6 (range: 22 - 76) years. Twenty-five were women (64%) and 14 were men (36%). The female to male ratio was 1.8 to 1. Histological classification according to WHO criteria was as follows: 34 cases (87%) were WHO grade I, from which 15 cases (38%) were psammomatous, 7 cases (18%) were meningothelial, 9 cases (23%) were transitional and 3 cases (8%) were fibroblastic. Five patients (13%) were grade II, 3 of which (7.5%) had clear cell appearance and the remaining 2 (5.5%) had chordoid appearance (Figures 1 and 2).
There was no significant difference in preoperative neurologic signs according to Frankel classification between different histological subtypes. Preoperative neurologic signs included pain, gait disturbance, paresthesia and bladder dysfunction. Table 2 shows the summary of pre- and post-operative neurologic deficits in 39 patients with spinal meningiomas.
| Preoperative Frankel Grade | Number of Patients | Postoperative Frankel Grade | ||
|---|---|---|---|---|
| Grade D - E | Grade C | Grade A - B | ||
| 31 | 25 | 6 | 0 | |
| 7 | 3 | 3 | 1 | |
| 1 | 0 | 1 | 0 | |
| 39 | 28 | 10 | 1 | |
Pre- and Postoperative Neurologic Deficits in 39 Patients With Spinal Meningiomas
Seventy-nine percent of patients had grade D and E, 19% grade C and 2% had grade B. Postoperatively 82% improved or not changed and 18% worsened. One patient with psammomatous cervical meningioma with Frankel grade C had severe grade B signs after surgery.
Following surgery, pain was relieved in all patients; bladder dysfunction (in one case) was cured. Paresthesia remained only in one out of ten patients. From 8 patients who were unable to walk (group B or C), five cases remained at the same status (group B or C) postoperatively from which two cases were psammomatous and three cases were WHO grade II meningioma.
When comparing surgical outcome between different histologic subtypes, we observed that 6 out of 15 patients with psammomatous meningioma (40%) had worsened results or persistent functional deficit of grade C or worse. All of five cases of WHO grade II meningioma worsened after surgery or remained unchanged in grade C. These are significantly different from remaining 19 cases of WHO grade I meningioma (non-psammomatous), all of which (100%) showed improved results or not changed post-operatively, remaining in grade D or E (P = 0.003 and P < 001) (Tables 3 and 4).
| Histologic Type | Number of Patients | Preoperative Grade | ||
|---|---|---|---|---|
| Grade D - E | Grade C | Grade A - B | ||
| Psammomatous | 15 | 12 | 3 | 0 |
| Fibroblastic | 3 | 2 | 1 | 0 |
| Meningothelial | 7 | 7 | 0 | 0 |
| Transitional | 9 | 8 | 1 | 0 |
| 5 | 2 | 2 | 1 | |
| 39 | 31 | 7 | 1 | |
Preoperative Neurologic Function and Histologic Subtypes
| Histologic Type | Number of Patients | Post-Operative Grade | ||
|---|---|---|---|---|
| Grade D - E | Grade C | Grade A - B | ||
| Psammomatous | 15 | 9 | 5 | 1 |
| Fibroblastic | 3 | 3 | 0 | 0 |
| Meningothelial | 7 | 7 | 0 | 0 |
| Transitional | 9 | 9 | 0 | 0 |
| 5 | 0 | 5 | 0 | |
| 39 | 28 | 10 | 1 | |
Postoperative Neurologic Function and Histologic Subtypes
The mean age of patients with good postoperative outcome was 51.8 while the mean age of patients with poor outcome was 51.5, so the age does not affect the outcome (P = 0.34).
Seven out of 11 patients with poor outcome (63.6%) and 18 of 28 patients with good outcome (64%) were women: sex had no significant influence on the outcome (P = 0.99).
Cervical location of tumor was seen in 5 out of 11 patients with poor outcome (45.5%) and in 3 out of 28 patients with good outcome (10.7%): cervical location had significant influence on the outcome (P = 0.027).
The mean tumor size was 2.7 cm in greatest dimension in patients with poor outcome and 2.6 cm in cases of good outcome (P = 0.40). Five of the poor-outcome cases have been reported to have adhesion during surgery and incomplete removal (45%), while two of the others have this condition (7%) (P = 0.012).
5. Discussion
Meningiomas are primary tumors of spines, arisen from the arachnoid cap cells of the meninges; they constitute about 1.2% of all CNS meningiomas (3). In our study, spinal meningiomas constitute 7.7% of CNS meningiomas; this could be due to the fact that Shohada hospital is a referral center for difficult cases of spinal tumor surgery.
5.1. Histopathology
Psammomatous subtype and grade II cases had poor postoperative outcome while the other subtypes of WHO grade I had good outcome in our study.
In Schaller study (11), psammomatous subtype had a less favorable outcome compared with other subtypes; in Roux et al. study (6), histological subtype did not seem to have any influence on the postoperative outcome.
5.2. Gender
Spinal meningiomas have high predilection for women; studies of Roux et al. (6), Klekamp and Samii (5), Haegelen et al. (4) and Sandalcioglu et al. (3) all revealed significantly higher incidence of meningioma in women as in our study that revealed a female to male ratio of 1.8 to 1. In the study of Roux et al. (6), as in our study sex had no influence on the prognosis.
5.3. Age
The average age of patients varies between 56 and 66 in studies of Roux et al. (6) and Haegelen et al. (4) respectively; in our study the average age was 51.6 and prognosis was not influenced by increasing age. In Schaller study age less than 60 years was correlated with a good outcome (11). In Sandalcioglu et al. study (3) elderly patients proved to harbor an increased risk for surgical morbidity. In our study the patients’ age had no influence on the post-operative outcome.
5.4. Anatomical Location
Cervical location of the tumor was related with poor outcome in our study; in Schaller study (11), tumor location below C4 seemed to be correlated with a good outcome; in other studies (3, 6, 10, 12) no correlation was found.
5.5. Completeness of Resection
Incomplete removal of the tumor was seen in about half of the cases with poor outcome and 7% of the other group. Since 80% of the incompletely removed tumors of the first group are of psammomatous subtype, it seems that incomplete removal of the tumor may be the reason of poor outcome in psammomatous subtype in comparison with other subtypes of WHO grade I, although further studies with larger sample size are needed to confirm this result.
5.6. Conclusion
Spinal meningioma of WHO grade I with psammomatous subtype are associated with less favorable postoperative neurologic outcome than other subtypes, with results closer to cases of WHO grade II. Incomplete resection of spinal meningioma occurs more frequently in psammomatous subtype and cases of WHO grade II and trying to complete resection may cause additional neurological damage. Further study with more patients is recommended for better evaluation of this issue. Cervical location is another factor that seems to have a negative correlation with a good outcome.