1. Background
Head and neck cancer has been the seventh most common malignancy and also a major cause of morbidity and mortality, worldwide (1, 2). Squamous cell carcinoma (SCC) has represented the most common histologic subtype of cancers originating from the region (3, 4). Cancers have been originating from oral cavity, larynx and pharynx comprised approximately 690,000 new cases and 4.9% of all cancer incidence through the world in 2012 (5). Unfortunately, most of the head and neck cancer patients have presented with loco-regionally advanced disease (6) and despite improvements in treatment techniques, the five years overall survival of such patients has been still poor (7) Several prognostic factors have defined for head and neck cancers that could influence response to therapy and eventual outcome. These factors might be categorized as follows: (a) prognostic factors related to the primary tumor like TNM stage, malignancy grading, perineural invasion, vascular invasion, (b) prognostic factors related to the patient like age, sex, general medical condition, and (c) prognostic factors related to the treatment (8). Currently, most oncologists favor mulimodality approach in the management of head and neck cancer, has focused on organ preservation, increasingly. Surgery, with or without adjuvant (chemo) RT and definitive (chemo) RT have been acceptable managements in most primary head and neck cancers and recent trials has shown improved survival rates with such approaches (9-11).
2. Objectives
The present study has conducted considering the influence of genetic and geographic factors on epidemiology of head and neck cancer and lack of reliable data on its prognosis and treatment outcome in Iran.
3. Patients and Methods
We have performed a retrospective analysis of 119 patients with non-metastatic non-nasopharyngeal squamous cell carcinoma of head and neck admitted between 2008 and 2014 to our clinical oncology center (Jorjani Cancer Center, Emam Hossein Hospital, Tehran, Iran). All medical records of the included patients have investigated and with a previously prepared fact sheet the following data have collected: age, sex, weight, height, date of pathologic diagnosis of the disease, primary tumor site, clinicopathological characteristics of the primary tumor, date of last follow up, date of recurrence and death (if any). The characteristics of the study population have then described and the overall survival (OS) and event free survival (EFS) of the patients and their relation with demographic and clinico-pathological factors have analyzed. This retrospective study has approved by the local scientific and ethical committee.
3.1. Statistical Analysis
Patients’ baseline characteristics, disease and treatment factors have summarized using descriptive statistics. The categorical parameters have compared using two-sided Pearson’s χ2 test or Fisher’s exact test, as appropriate. The event free survival (EFS) has defined as survival without progression, recurrence, and death. The overall survival (OS) time has defined as the period from the diagnosis until death of any cause or until the date of the last follow-up, at which data point has censored. All summary statistics on time-to-event variables have estimated according to the Kaplan-Meier method and compared using the log-rank test. SPSS software (version 21.0) has used for statistical analysis. A P value < 0.05 has considered significant.
4. Results
4.1. Patient, Tumor, and Treatment Characteristics
Among the 119 studied patients, 90 were male (76%) and 29 were female (24%) with mean age of 58 years (16 to 88 years). Most patients (59% of all) had normal body mass index (BMI). Larynx was the most common primary tumor site (55% of all patients) and oral cavity had the second place subsequently. According to primary tumor and lymph node stage, most cases were T1/T2 (54%) and N0/N1 (50%), respectively. Treatment has consisted of surgical (surgery + RT/ChemoRT) and non-surgical (RT/ChemoRT or Induction Chemo + RT/ChemoRT) modalities. The patient, tumor and treatment characteristics have detailed in Table 1.
| Variables | Values |
|---|---|
| ≤ 50 | 31 (26) |
| > 50 | 88 (74) |
| Female | 29 (24) |
| Male | 90 (76) |
| Bellow Normal | 14 (12) |
| Normal | 70 (59) |
| Above Normal | 35 (29) |
| Larynx | 65 (55) |
| Oral Cavity | 35 (29) |
| Hypopharynx | 15 (13) |
| Other | 4 (3) |
| T1/T2 | 64 (54) |
| T3/T4 | 52 (44) |
| Missing | 3 (2) |
| N-negative | 39 (33) |
| N-positive | 73 (61) |
| Missing | 7 (6) |
| N0/N1 | 59 (50) |
| N2/N3 | 53 (44) |
| Missing | 7 (6) |
| Surgical | 62 (52) |
| Non-surgical | 41 (35) |
| Missing | 16 (13) |
The Patient, Tumor and Treatment Characteristicsa
4.2. Survival Analysis
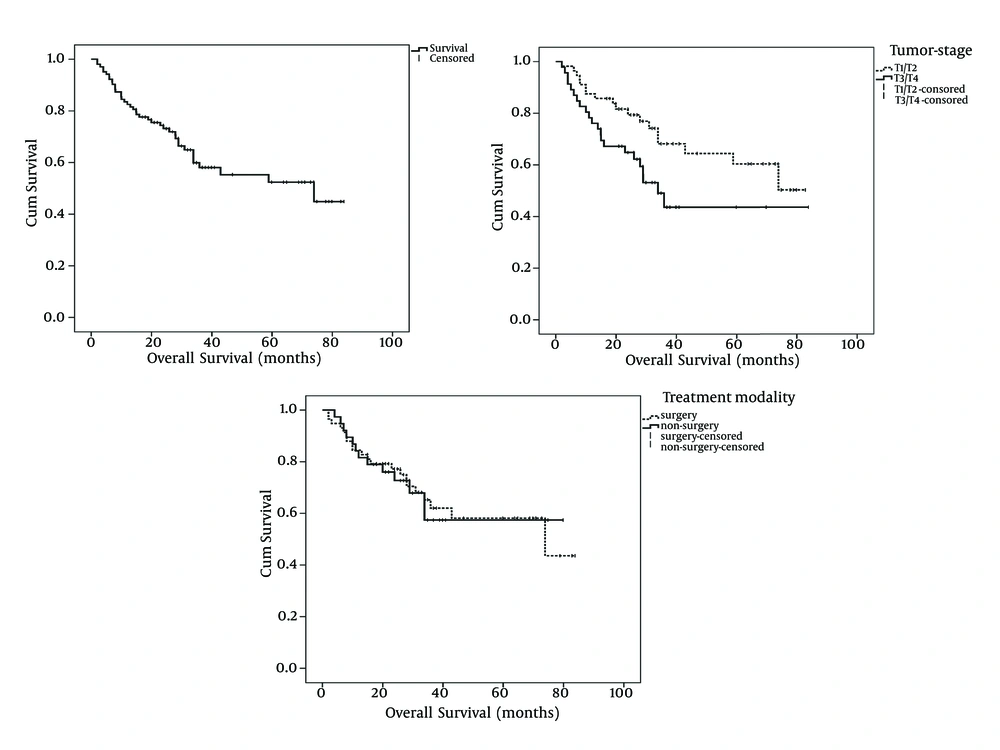
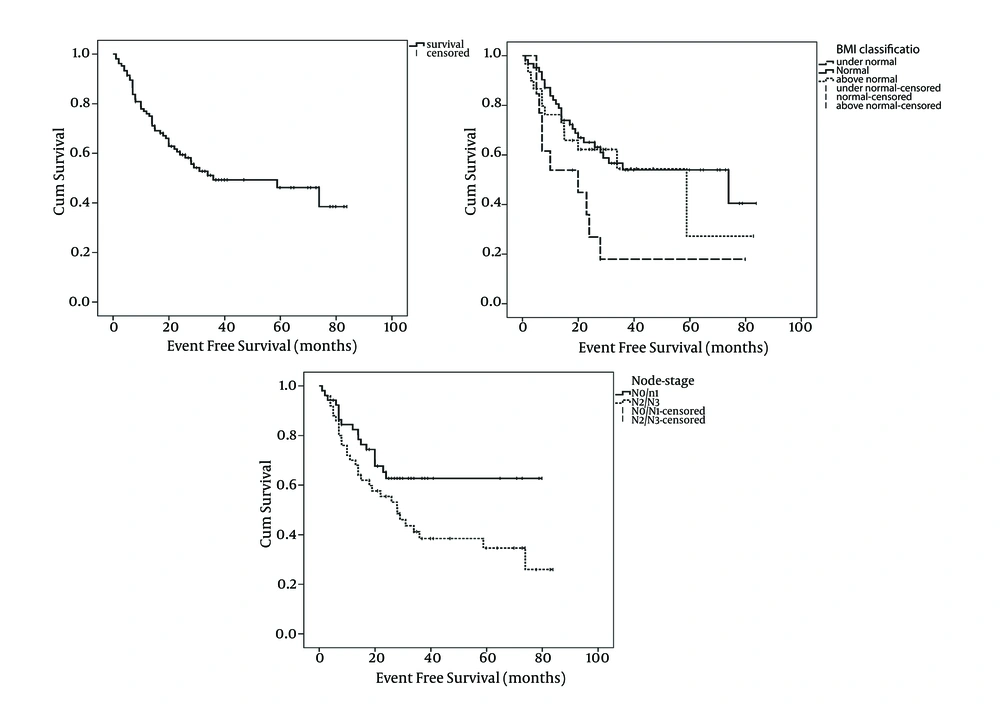
With a median follow-up period of 28 months (range: 2 to 84 months), OS and EFS of the study patients was 61.2% and 52.4%, respectively (Figures 1 and 2). Tumor stage (T-stage) was the only parameter that significantly influenced the patients’ OS. Mean OS of patients with T1/T2 tumors was higher than patients with T2/T3 tumors and this difference was statistically significant (59 months vs. 47 months, respectively: P = 0.039). In terms of EFS, significant differences have observed among node stage (N-stage) and BMI classification subgroups. Patients with N2/N3 tumors had lower mean EFS than patients with N0/N1 tumors (40 months vs. 55 months, respectively: P = 0.043). Interestingly, the patients with normal BMIs had significantly higher mean EFS compared with patients with bellow or above normal BMIs (Table 2 and Figure 2). Patients whose primary tumor site was larynx had higher mean EFS and OS than patients with non-laryngeal tumors although the difference had trends toward statistically significance only in terms of EFS (Table 2). The correlation between survival and patient, tumor and treatment characteristics have detailed in Table 2. As shown in the Table 2, surgical treatment modalities have resulted in the same prognosis as non-surgical approaches.
| Variables | No | Mean OS, mon | P Value | No | Mean EFS, mon | P Value |
|---|---|---|---|---|---|---|
| 0.785 | 0.425 | |||||
| Female | 23 | 49 | 24 | 42 | ||
| Male | 80 | 55 | 81 | 48 | ||
| 0.130 | 0.779 | |||||
| ≤ 50 | 28 | 61 | 29 | 50 | ||
| > 50 | 75 | 51 | 76 | 46 | ||
| 0.808 | 0.035 | |||||
| Bellow normal | 13 | 46 | 13 | 26 | ||
| Normal | 61 | 56 | 62 | 51 | ||
| Above normal | 29 | 47 | 30 | 45 | ||
| 0.417 | 0.096 | |||||
| Larynx | 57 | 59 | 58 | 54 | ||
| Non-larynx | 46 | 49 | 47 | 40 | ||
| 0.039 | 0.742 | |||||
| T1/T2 | 56 | 59 | 57 | 48 | ||
| T3/T4 | 46 | 47 | 47 | 45 | ||
| 0.101 | 0.043 | |||||
| N0/N1 | 50 | 57 | 52 | 55 | ||
| N2/N3 | 50 | 49 | 50 | 40 | ||
| 0.930 | 0.654 | |||||
| Surgical | 58 | 56 | 59 | 48 | ||
| Non-surgical | 38 | 54 | 39 | 50 |
Correlation Between Survival and Patient, Tumor and Treatment Characteristicsa
5. Discussion
In the present study, patients’ demographic characteristics like age and sex were similar to ones in other studies (both local and in other geographic regions of the world) (1-3). Larynx was the most common primary site followed by oral cavity and this finding has been in concordance with most other reports (1-3, 12). 28 Months OS and EFS of our patients was 61.2% and 52.4%, respectively. In a study on incidence and survival trends of head and neck squamous cell carcinoma in the Netherlands, Braakhuis et al. has shown a 2-year survival rate of 72% for the patients had been diagnosed between 2007 and 2011 (13). Oropharyngeal carcinoma has constituted the significant proportion of their head and neck cancers and its relation to HPV-positivity could contribute to the better prognosis has observed in their study population. Other possible reasons for the disparity between the survival rate has observed in their study and one in our investigation could be higher socioeconomic status of their patients and better access to health care facilities that resulted in earlier diagnosis and treatment of the cancer. In another study on prognosis of the patients with head and neck cancer, Dwojak et al. has shown a survival rate of 62% for American Indians at two years (14). This finding was very similar to one observed in our study and could also be explained with previously mentioned reasons. In our patients T-stage and N-stage have demonstrated to be significantly associated with survival and these findings are consistent with results of most other studies (15-17). As an interesting observation in the present study, patients with normal BMIs had significantly higher mean EFS in comparison with patients with bellow or above normal BMIs. Nutrition has been a matter of concern in cancer (especially head and neck cancer) management. Obesity was one of well-known risk factors for cardio-vascular disease and has also related to an increased risk of cancer progression and death (18). On the other hand, malnutrition has shown to be a risk factor of worse prognosis in some types of cancer, including HNSCC (19, 20). In a retrospective study has included 706 patients with head and neck cancer diagnosed between 2004 and 2012, Takenaka et al. has shown that BMI was a prognostic factor for survival, independent of primary site, and tumor stage. Patients with normal pre-treatment BMIs had higher 5-year survival rate in comparison with underweight patients and this difference was statistically significant (62.7% vs. 32.2%, respectively P < 0.001) (20). In terms of treatment modality, our study had also a remarkable result. Patients whose primary treatment has included surgery had the same prognosis as ones with non-surgical approaches (Figure 1, Table 2). There were some evidence in the literature that has shown similar outcomes for head and neck cancer patients treated with surgical and non-surgical approaches (21, 22). In a recently published randomized trial, Iyer et al. has shown that there have appeared to be either no difference in outcome, nor a slight advantage favoring primary surgery plus RT compared with concurrent ChemoRT for patients with advanced, nonmetastatic squamous cell carcinoma of the head and neck (5-year OS rate was 45% and 35%, respectively P = 0.262). Only in subset analysis, patients with oral cavity cancer had significantly better prognosis with surgical approaches (22). In conclusion, our study seems to be the first that investigated outcome of Iranian patients with head and neck cancer and factors influencing it.