1. Background
Breast cancer has been one of the most common cancers, and the main women death cause all over the world (1-3). For prognostic and treatment purposes, breast cancer has been categorized based on histological type, size of tumor, metastasis, ER, PR, HERB2 expression and lymph node involvement (4, 5). Breast cancer is a heterogenous disease because patients with the same diagnostic and clinical prognostic profile have shown different clinical outcomes. This difference is possibly caused by the limitation of current taxonomy of breast cancer, which is mainly based on morphology (5). To overcome this problem, new efforts are underway to find new and more accurate biological markers to improve the current calssification.
Integrins are a family of heterodimeric transmembrane receptors of cell adhesion molecules (6). Integrins participate in cell-cell adhesion and are of great importance in interactions of cells with components of the extracellular matrix such as fibronectin (Fg), fibrnectin (FN) and collagen (Col). After binding to extra cellular matrix or other cells, integrins activate MAPK pathways that regulated different cell activities like differentiation, migration, immune response and cell morphogenesis (6). Cell-extracellular matrix (ECM) interactions plays a fundamental role in cell growth, organ development, tissue regeneration, and wound healing as well as in malignant growth processes (7).
Fg is a 340 kDa protein is produced in liver. This protein is involved in blood coagulation and prevents bleeding. After binding to integrin αIIbβ3 on platelet membrane, Fg binds platelets group to endothelium of blood vessels. The best-known integrin binding to Fg is αMβ2 that is located on leukocyte membrane (8, 9). FN exists as a soluble protomeric form in blood serum and insoluble multimeric form in ECM. FN is nonreactive with adhesion receptors in its soluble form but in its insoluble form is highly adhesive (10). FN polymerization in ECM is highly regulated to produce correct binding domains on ECM (11, 12).
2. Objectives
Since a better understanding of MEK/ERK pathway or complex signaling regulation in breast cancer especially in metastasis is necessary for finding new biomarkers or assess prognosis and drug response, here we have studied the p-ERK, p-MEK, p-Src and p-FAK expression in breast cancer cell lines cultured in plates pre-covered with substrates (FN, Fg, and collagen).
3. Materials and Methods
3.1. Antibodies
Antibodies against ERK, MEK and the HRP secondary antibodies were obtained from Santa Cruze biotechnology; αvβ3 (LM609) was from Chemicon; phospho-ERK, MEK and phospho-MEK (9121) were from cell signaling. Fibronectin, collagen, and fibrinogen were obtained from sigma.
3.2. Cell Culture
All the cell lines were from ATCC and have cultured in RPMI-1640 containing 10% FCS and 100 U/mL penicillin/streptomycin. Cells were grown at 37°C in a humidified incubator with 5% CO2 and 95% air.
3.3. Flow Cytometry
Cells were grown in 100 mm dish (VWR) to about 95% confluency and harvested with 2% EGTA. Cells were washed and re-suspended in incubation buffer (IB) (13 mM NaCl, 2.7 mM KCl, 3.3 mM NaH2PO4, 3.8 mM Hepes buffer, 1 mM MgCl2, 5.5 mM Glucose and 1 mg/mL BSA). Cells were incubated with first antibody at r/t for an hour, washed three times with IB and incubated with Alexa Fluore 488 labeled secondary antibodies for 30 m at r/t. cells were washed and analyzed with a FACScan flowcytometer Coulter® Epics®XL. (The acquisition software was Beckman Coulter EXPO32). Data were analyzed using Flowjo program and Excel program was used to draw the graphs.
3.4. Cell Culture and Morphology
Cells were cultured in plates precovered with different substrtes (Collagen, fibrinogen, fibronectin) and the morphology of the cells was observed under a invert phase contrast microscope at 30 minutes, 1 hour, 3 hours, 5 hours and 1 day of incubation.
3.5. Western Blotting
After one hour incubation of cell lines on different substrates, cell plates were washed once with cold PBS and lysed with 500 µL of RIPA buffer (50 mM Tris, pH 8, 150 mM NaCl, 0.1 % SDS, 0.5 % Na deoxycholic Acid, 1% NP-40 or IGEPAL, 10 µg Aprotinin per ml, and 10 µg Leupeptin per mL), after leaving the plates on ice for 10 Min, cells were scrapped and broken down with a 25G 5/8 needle. The cell extract was centrifuged for 30 m at 14,000 rpm and the supernatant was kept at -20°C. Protein concentration was assayed with Lowry kit from sigma. Equal amounts of protein (24 µg/well) were electrophoretically separated in SDS polyacrylamide gels and proteins were transferred onto nitrocellulose membrane with a semidry gel transfer apparatus. Membranes were blocked with 5% milk in PBST for 1 - 2 hours at r/t. Membranes were incubated with primary antibody at 4°C for o/n. Then, membranes were washed for 10 minutes with PBST and incubated with HRP-labeled antibody in 5% milk in PBST for 1 hour. Membranes were washed for 20 - 30 minutes with PBST, treated with chemiluminescence reagents and exposed to Kodak film.
4. Results
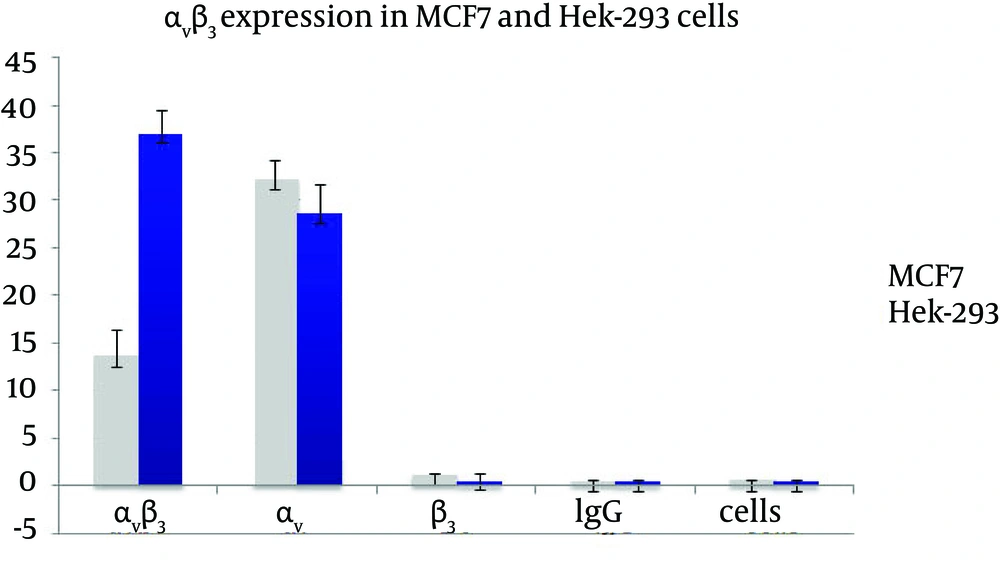
Flow cytometry results showed that Hek-293 cells had higher level of αvβ3 expression than MCF7 cells but both had a low level of αv and β3 subunites (Figure 1).
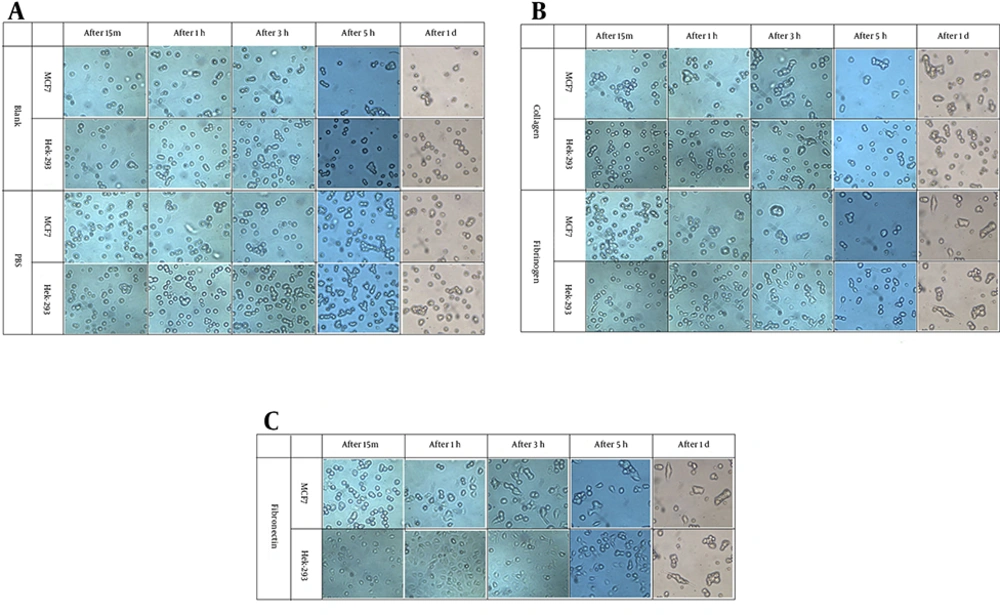
Morphology of the cells: the morphology of the cells were compared in plates precovered with different substrtaes after different period of time (15 minutes, 1 hours, 3 hours, 5 hours, and 1 day).
In blank plates (with no extra substrate) and PBS (the solvent of the substrates) most of the cells were floating in the cell culture media and only after a day, there was sign of attachment (Figure 2A). The attached cells were nicely spread out but the unattached cells were either dome shape or floating in the media. In comparison, there were more attached MCF7 cells than Hek-293 cells in corresponding blank plates (Figure 2A).
In Col precovered plates, MCF7 cells were attached better than Hek-293 cells to Col, since, MCF7 cells have shown the sign of attachmnet after 15 minutes but Hek-293 cells started to attach after 3 hours (Figure 2B). In contrast, in Fg plates Hek-293 cells have attached better than MCF7 cells (Figure 2B). In Fg precovered plates, Hek-293 cells started to attach after 15 and the binding improved by incubation time while MCF7 cells showed the sign of attachment after one hour and even after 1 day they did not attach to the substrate like Hek-293 cells (Figure 2A to 2C). Both cell lines started to attach to FN precovered plates after 15 min but Hek-293 cells attached better than MCF7 cells over incubation time (Figure 2A to 2C).
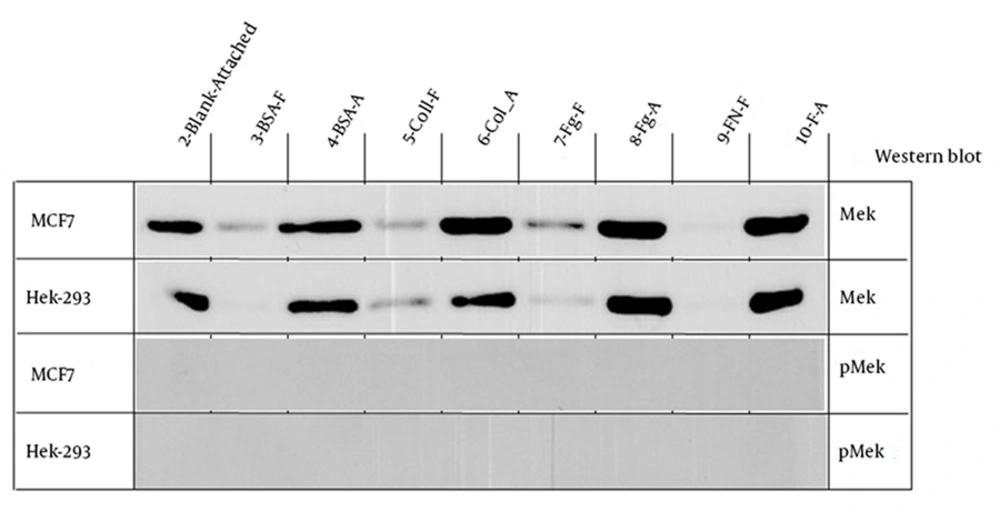
4.1. MEK and p-MEK
Both cell lines showed strong signal for MEK expression in cells that were attached to different substrates but a weak signal for the free cells in BSA, Col, and Fg plates (Figure 3). However in comparison MCF7 cells showed a stronger signal for the free cells in Fg precovered plates (Figure 3). Neither of the cell lines showed any signal for p-MEK in attached or free cells in different precovered plates.
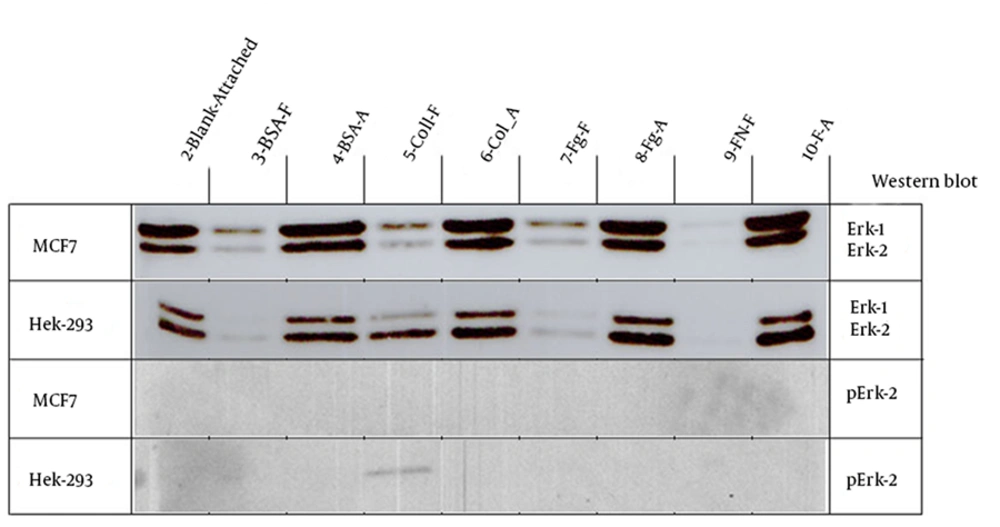
4.2. ERK and p-ERK
Although both cell lines showed a strong signal for ERK expression in attached cells but MCF7 cells showed stronger signal than that of Hek-293 cells (Figure 4). In MCF7 cells the free cells in BSA, Collagen and Fg showed a weak signal for ERK expression but in Hek-293 cells only free cells in collagen plates showed the same signal for ERK expression (Figure 4). Overall, ERK expression in MCF7 cells was stronger than that in Hek-293 cells (Figure 4). Regarding p-ERK expression, none of the celll lines showed any signal except for the free Hek-293 cells in collagen plate that showed a weak signal (Figure 4).
5. Discussion
This study has designed to compare the MEK/ERK signaling pathway in MCF7 and Hek-293 cells cultured in pre-covered plates with different substrates (FN, Fg, Col). After one hour incubation the free and attaches cells were separated, washed and total protein was extracted for each one. The expression of signaling proteins; ERK, p-ERK, MEK and p-MEK, were assayed with immunobloting.
Although in both cell lines the attached cells showed a strong signal for ERK expression but the level of ERK1 and ERK2 was different in these two cell lines. In MCF7 cells the expression of ERK1 (44 kDa) was stronger than ERK2 (42 kDa) but in Hek-293 cells the expression of ERK2 was higher than ERK1. Two isoforms of ERK in vertebrates are ERK1 and ERK2 which are 84% identical at amino acid level (13). ERK1 and ERK2 are very similar and ubiquitously expressed, however, their relative distributions across tissue is different (13). Despite the similarities between these two isoforms, the invalidation of ERK1 or ERK2 has indicated clear difference between them. The invalidation of ERK2 has resulted in placental defect and embryonic death at day 6.5 (14-16) in contrast mice lacking ERK1 has lived and reproduced normally (17, 18) with minor defects such as terminal differentiation of T lymphocytes (18), decreased adiposity with fewer adipocytes (19), and facilitated learning and memory (20). In a study Vantaggiato et al. has proposed that ERK1 and ERK2 had opposite roles in Ras-mediated signaling, with ERK1 antagonizing the positive signaling has provided by ERK2 (21). The different pattern of ERK1 and ERK2 expression might provide useful information to differentiate the two cell lines or cancer from normal cells.
No phospho-ERK was detected in cells cultured in different plates except a weak signal for free cells in collagen precovered plates. Both cell lines showed a strong signal for MEK expression in all the attached cells. The MCF7 cells attached to Col, Fg or FN had the strongest signal while the Hek-293 cells attached to Fg and FN showed the strongest signal. In comparison, MCF7 cells attached to fibrinogen showed a stronger signal than that of Hek-293 cells.
No phospho-MEK was detected in cells cultured in different plates. Protein phosphorylation presentsa mechanism to switch on or off a protein activity (22-24). Protein phosphatases have the task of undoing phosphorylation (25). Constitutive activation of MAPK has been detected in several cancer cell lines and tumors (26, 27). Our results showed that, after cell attachment all p-ERK or p-MEK have been deactivated. Since we have incubated the cell lines for one hour before protein extraction, there might be an ERK or MEK activation in the beginning of cell attachment, but after 1 hour became deactivated again. Additional experiments with shorter incubation times might show the activated ERK (p-ERK) or MEK (p-MEK) in attached or free cells.
The free Hek-293 cells in collagen plates showed a weak signal for ERK and also for phospho-ERK protein. Hek-293 cells had high affinity to adhere to collagen. This is the reason that precoverd plates with collage or poly-L-lysine are being used to increase Hek-293 cells adherence to cell culture plates. In our study Hek-293 cells showed a betetr attachment to collagen than MCF7 cells. The higher attachment of Hek-293 cells to collagen has been reported before (28). Our results showed that Hek-293 cells had higher level of αvβ3 integrin than MCF7 cells. Previous studies have shown a higher level of αvβ3 expression in Hek-293 cells, compared to MCF7 cells as well (29). Hek-293 cells binding to Col have been reported to be integrin-related since using anti-integrin blocking antibodies was able to completely inhibit the attachement (30). The change of integrin expression following malignant transformation might have a key role in defining invasive behavior of a tumor. The best known integrin binding to collagen belongs to β1 integrin subfamily. There are four colagen binding integrins in mammalian cells: α1β1, α2β1, α10β1and α11β1 (31-33), however none of these integrins been assayed in Hek-293 cells in this study.
Signaling pathways like ERK/MEK/RAF and integrins have shown important roles in cell behavior like binding to different substrate or migration. Therefore, studying signaling proteins like ERK or MEK in unattached (free) or attached cells to different substrates might help to find a better target protein to prevent cancer metastasis.