1. Context
Chronic myeloid leukemia (CML) is a myeloproliferative disorder characterized by overproduction of immature and mature myeloid cells in the peripheral blood, bone marrow and spleen. In more than 90% of cases, the disease is diagnosed during the initial chronic phase (CML-CP), which is characterized by expansion of functionally normal myeloid cells. If untreated, CML progresses to an initial accelerated phase (AP), and subsequently to a more aggressive blast phase (BP), with loss of terminal differentiation capacity. A hallmark of CML is the presence of (9; 22) (q34; q11) reciprocal translocation, which is cytogenetically visible as Philadelphia chromosome (Ph) and results in the formation of BCR-ABL1 fusion protein. This fusion protein is a constitutively active tyrosine kinase which is necessary and sufficient for malignant transformation (1). In vitro studies have demonstrated that BCR-ABL1 is oncogenic, and leads to leukemic cell proliferation and inhibition of apoptosis (2). It is believed that BCR-ABL1 gene is initially generated in a single hematopoietic stem cell (HSC) which gives it proliferative advantage over its normal counterparts, eventually leading to an expanded myeloid compartment (3).
2. Evidence Acquisition
The introduction of imatinib, a BCR-ABL1- targeting tyrosine kinase inhibitor (TKI) has revolutionized CML therapy. Following the success of the pivotal IRIS (international randomized study of interferon and STI571) trial, imatinib mesylate (Gleevec, Novartis, Basel, Switzerland) - formerly known as STI571- rapidly became the preferred first line treatment for patients with newly diagnosed CML in chronic phase (4, 5). Subsequently, two other novel TKIs with increased activity against BCR-ABL1 were developed, dasatinib (Sprycel, Bristol-Myers Squibb, Princeton, NJ) and nilotinib (Novartis), which were approved for newly diagnosed CML patients and those with previously treated CML (6, 7). Another BCR-ABL1 inhibitor is bosutinib (Tasigna, Pfizer, New York, NY) which has been approved for the treatment of chronic, accelerated, or blast phase of CML (8). Ponatinib (Iclusig, ARIAD, Cambridge, MA) is a potent multitargeted kinase inhibitor that has been approved for the treatment of CML-CP, CML-AP, and CML-BP (9). Nevertheless, CML therapy faces major challenges.
The first is the development of resistance to BCR-ABL1 inhibitors in some patients, which can be due to BCR-ABL1 overexpression, differences in cellular drug influx and efflux, activation of alternative signaling pathways, or emergence of BCR-ABL1 kinase domain mutations during TKI treatment (10).
The second is the limited efficiency of BCR-ABL1-TKIs in blast crisis (BC) CML (11). This can be due to generation of additional chromosomal and molecular changes during transition from chronic phase to blast phase. Therefore, these CML blast cells may not depend entirely on BCR-ABL1 pathway for survival (12, 13). Targeting additional pathways may be necessary for treating advanced CML.
The third is the insensitivity of CML stem cells to BCR-ABL1 inhibitors (14, 15). CML is sustained by a population of CD34+/ BCR-ABL1+ progenitor cells with stem cell properties. One of the characteristics of CML stem cells is that they are quiescent. Therefore, conventional chemotherapeutics and BCR-ABL1 inhibitors which act by inhibiting cell proliferation and inducing apoptosis, are ineffective against these non-proliferating stem cells (16, 17). To reach an ultimate cure, development of new and more effective therapies involving elimination of CML stem cells is required.
3. Results
3.1. BCR-ABL1 Signaling Pathway
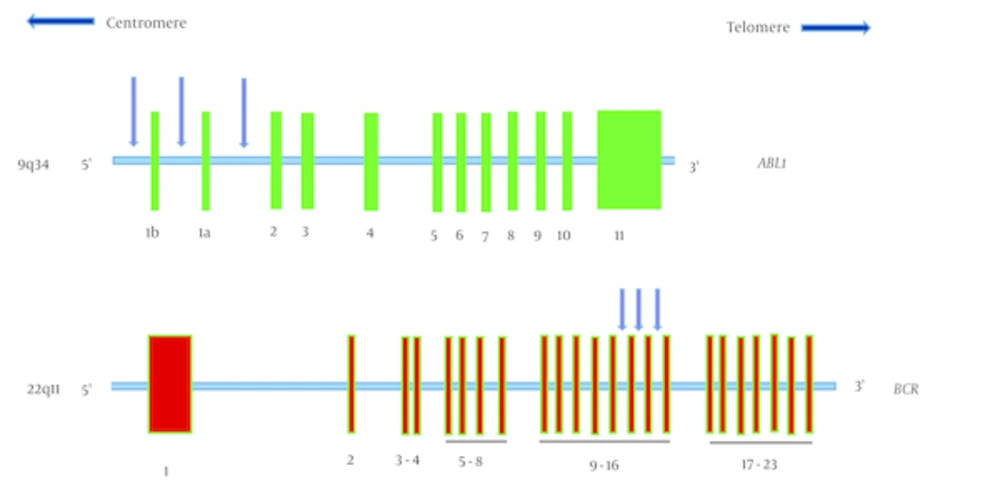
The breakpoints within the ABL1 gene occurs either upstream of exon 1b, downstream of exon 1a, or more frequently, between exons 1b and 1a (Figure 1 A). In most patients with CML, the breakpoints within the BCR gene take place in a 5.8-kilobase area spanning exons 12 - 16, referred to as the major breakpoint cluster region (M-BCR) (Figure 1 B) (1).
1a, The ABL1 gene is located on chromosome 9q34 and spans more than 230 kb. It contains two alternative first exons, exon 1b and 1a, followed by exons 2 to 11. Exon 1b is approximately 200 kb upstream of exon 1a. The breakpoints that create the Philadelphia chromosome, are scattered over a large area (more than 300 kb) at the 5’ end of the gene, either upstream of alternative exon 1b, between alternative exons 1b and 1a, or between exons 1a and 2. The BCR gene is located on chromosome 22q11 and spans approximately 135 kb. This gene contains 23 exons. In most patients with chronic myeloid leukemia, the breakpoint is in the major breakpoint cluster region that spans exons 12 - 16.
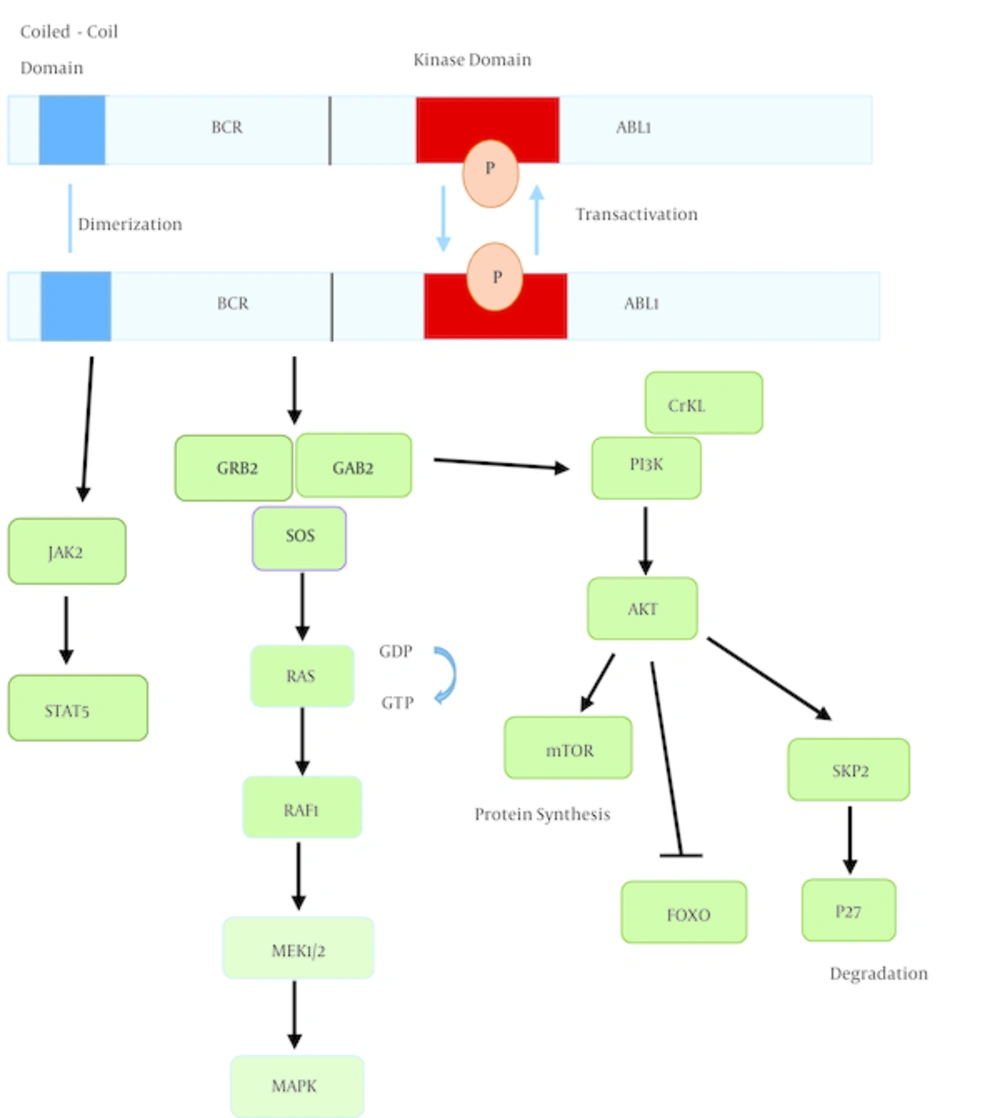
The N-terminal coiled-coil domain of BCR-ABL1 has dimerization and trans-autophosphorylation activity (18). Autophosphorylation of tyrosine-177 of BCR-ABL1 results in formation of a complex between GRB2, GAB2, and SOS, leading to activation of RAS and phosphatidylinositol 3-kinase (PI3K) pathways (19). Signaling from RAS-RAF-MEK-MAPK pathway enhances proliferation. PI3K activates the serine-threonine kinase AKT, which results in: 1, promoting survival by inhibiting FOXO transcription factors (20); 2, activation of mTOR which increases protein translation (21); and 3, increasing the expression of SKP2, the recognition protein of SCFSKP2 E3 ubiquitin ligase, which degrades p27 and results in enhanced proliferation (22). Another crucial consequence of BCR-ABL activation is STAT5 activation, either through direct phosphorylation or indirectly through phosphorylation by JAK2 or HCK (23). Lack of STAT5 inhibits both myeloid and lymphoid leukemogenesis (24) (Figure 2).
Dimerization of BCR-ABL1 induces autophosphorylation and activation of the kinase, generating docking sites for adaptor proteins like GRB2. This signaling pathway results in activation of multiple downstream targets, leading to increased proliferation, enhanced survival, and perturbation of cell adhesion and migration.
3.2. Mechanism of Action of BCR-ABL Inhibitors
Imatinib, dasatinib, and nilotinib bind to the ATP-binding pocket of the ABL kinase domain and inhibit BCR-ABL activity (25-27). Imatinib binds to the inactive conformation of BCR-ABL, and prevents conformational changes needed for BCR-ABL activation (25). Nilotinib was developed by changing the structure of imatinib and binds to BCR-ABL with a higher affinity. Nilotinib is 10 to 50 times more potent than imatinib for inhibiting BCR-ABL (26). Dasatinib has a different chemical structure and binds to a slightly different region of the ATP- binding pocket. Dasatinib binds to the active conformation of BCR-ABL and is 325 fold more potent than imatinib and 16 fold more potent than nilotinib (27).
3.3. Cytogenetic and Molecular Response During CML Treatment
Several different methods are used to assess cytogenetic or molecular response during CML treatment. Complete hematologic response is assessed by analysis of peripheral blood counts. Conventional cytogenetic analysis of bone marrow aspirates is performed by assessing Ph positivity in at least 20 Giemsa- stained metaphase cells. Complete cytogenetic response (CCyR) is defined as the absence of detectable Ph+ metaphase cells, which is a clinically important prognostic marker in CML patients. In patients without complete cytogenetic response, the degree of cytogenetic response is classified as partial (1% - 35% Ph+ metaphase cells), major (0% - 35% Ph+ metaphase cells), or minor (> 35% Ph+ metaphase cells). A limitation of cytogenetic testing is the need for an invasive bone marrow biopsy to culture cells and obtain metaphase spreads. Furthermore, the limit of detection of this method is relatively low, because a minimum of 20 metaphase cells are analyzed (1:20 or 5%). However, cytogenetic testing has the advantage of detecting, in addition to Philadelphia chromosome, other chromosomal abnormalities which may have prognostic value (28).
A more sensitive detection method is to assess peripheral blood interphase cells with fluorescence in situ hybridization (FISH), thereby analyzing a higher number of cells in comparison with conventional cytogenetic testing. Approximately, 100 to 500 interphase cells are usually assessed with FISH, so this method is more sensitive (1:500 to 1:100, or 0.2% to 1%). However depending on the probes used, FISH can have a false-positive rate of 1% to 10%. FISH method can be used for monitoring response to therapy until FISH levels are less than 5% to 10%. This method is not useful for analyzing further reduction in Ph+ metaphases (28).
Real-time quantitative PCR is an effective and clinically validated method to quantify BCR-ABL1 transcript levels and assess molecular responses. Guidelines from national comprehensive cancer network (NCCN) and European LeukemiaNet (ELN) recommend serial BCR-ABL1 RT-qPCR assays at regular 3 to 6-month intervals for routine minimal residual disease (MRD) monitoring of CML treatment (28, 29). Molecular monitoring includes RNA extraction from a bone marrow or peripheral blood sample and subsequent RT-qPCR to measure the level of BCR-ABL1 transcripts relative to a reference gene. In comparison with cytogenetic testing, PCR-based molecular monitoring offers exquisite sensitivity, almost 100 to 1000 times greater than FISH or conventional cytogenetic analysis. It can assess bone marrow or peripheral blood samples, and provides quantitative results with validated clinical response thresholds (30, 31).
3.4. Mechanisms of Resistance to TKI Inhibitors
A significant proportion of CML patients do not achieve a satisfactory response to first-line TKI therapy, which is mostly due to drug resistance. TKI resistance is classified as primary (lack of initial response to TKI) or acquired (loss of response). Primary imatinib resistance is defined as failure to achieve any of the following criteria: a complete hematologic response (CHR) within 3 to 6 months, any cytogenetic response (CyR) by 6 months, a major cytogenetic response (MCyR) by 12 months or a complete cytogenetic response (CCyR) by 18 months on imatinib treatment. Primary hematologic resistance is very rare in newly diagnosed CML-CP patients, whereas primary cytogenetic resistance is seen in 15% to 25% of patients. Suboptimal response is defined as: no CyR at 3 months, less than MCyR at 6 months, less than CCyR at 12 months, or less than major molecular response at 18 months. Suboptimal response identifies patients at risk of imatinib resistance. Acquired resistance is defined as loss of CHR or CCyR or progression of CML at any time on treatment (28, 29, 32). It has been shown that the risk of imatinib resistance varies depending on CML phase: the risk is lowest in CML-CP and highest in CML-BP (4, 33).
Mutations in the ABL1 tyrosine kinase domain of BCR-ABL1 are responsible for a substantial proportion of imatinib resistance. BCR-ABL1 mutations have been detected in 35% - 45% of CML patients with imatinib resistance (4, 34, 35). These mutations are more frequently found in patients with acquired resistance rather than primary resistance (36). Furthermore, BCR-ABL1 mutations occur more often in patients with advanced CML compared to CML-CP (37).
Less frequently, amplification of BCR-ABL1 gene or increased BCR-ABL1 expression can also lead to TKI resistance (34, 38). BCR-ABL1 independent mechanisms include activation of the SRC family of kinases, cytogenetic clonal evolutions with emergence of additional chromosomal abnormalities in Ph+ CML cells, or the presence of quiescent stem cells may also contribute to TKI resistance (34, 39). Different mechanisms proposed to be involved in TKI resistance are summarized in Table 1.
| Poor compliance |
| Poor intestinal absorption |
| Drug interactions |
| Heterogeneity of CML cells |
| Reduced drug influx |
| Increased drug efflux |
| Clonal evolution |
| Quiescent stem cells |
| Increased BCR-ABL1 expression |
| ABL1 kinase domain mutations |
3.5. ABL1 Kinase Mutations
Point mutations in the ABL1 kinase domain are the most frequent mechanism of TKI resistance. These mutations impair drug binding by changing essential amino acids for direct contact with the TKI or by preventing BCR-ABL1 from adopting the inactive conformation appropriate for TKI binding. The T315I mutation is one of the most frequent mutations detected in 4% to 19% of resistant cases (37, 40). This mutation confers resistance to all BCR-ABL1 kinase inhibitors. Some investigators have suggested that T315I is associated with disease progression and poor survival (40, 41)
Different mutations in the ABL1 kinase domain have been classified into four categories: a, in the imatinib binding site; b, in the P-loop (ATP binding site); c, in the catalytic domain; d, in the activation (A) loop. Mutations in the P-loop (residues 244-255 of ABL1) accounts for about 48% of all mutations in imatinib resistant cases, a worse prognosis regardless of sensitivity to imatinib (42-44). Mutations in the catalytic domain (residues 350 - 363 of ABL1) can also affect imatinib binding. The activation loop is the major regulatory component of ABL1 kinase domain and can adopt an open-active or closed-inactive conformation. Mutations in the activation loop impair adopting the inactive conformation which is required for imatinib activity. Amino acid substitutions at seven residues [M244V, G250E, Y253F/H, E255K/V (P-loop), T315I (imatinib binding site), M351T, and F359V (catalytic domain)] account for 85% of all resistance associated mutations (36, 45).
Although point mutations have more frequently been detected in TKI resistant and advanced CML, they have also been detected prior to TKI treatment. These findings suggest that prior to TKI therapy; these mutations do not confer a survival advantage (46). It is unclear whether certain mutations are responsible for CML progression or they are a consequence of genomic instability in advanced disease (47). It seems that gain of function mutations may contribute to CML progression, whereas loss of function mutations are frequently subject to selective pressure by imatinib (48, 49).
Table 2 summarizes in vitro sensitivity of BCR-ABL1 kinase domain mutations to imatinib, nilotinib and dasatinib. Although nilotinib and dasatinib inhibit most of the clinically relevant ABL1 mutations, with the exception of T315I mutation, the presence of mutations after imatinib failure as well as development of new mutations on subsequent TKI treatment is a potential source of resistance to successive TKI (50-53). It has been shown that in imatinib-resistant patients, subsequently treated with an alternative TKI, 83% of cases after an initial response are associated with emergence of newly acquired mutations. Patients already harboring imatinib-resistant kinase domain mutations, had higher likelihood of relapse associated with development of further mutations compared to patients who did not have mutations (54).
| BCR-ABL1 Mutation | Location | Imatinib | Nilotinib | Dasatinib |
|---|---|---|---|---|
| P-loop | Intermediate | Intermediate | Resistant | |
| P-loop | Intermediate | Sensitive | Sensitive | |
| P-loop | Intermediate | Intermediate | Intermediate | |
| P-loop | Resistant | Intermediate | Sensitive | |
| P-loop | Resistant | Intermediate | Intermediate | |
| ATP binding region (Drug contact site) | Sensitive | Sensitive | Resistant | |
| ATP binding region (Drug contact site) | Sensitive | Sensitive | Sensitive | |
| ATP binding region (Drug contact site) | Resistant | Resistant | Resistant | |
| ATP binding region (Drug contact site) | Sensitive | Intermediate | Intermediate | |
| ATP binding region (Drug contact site) | Intermediate | Sensitive | Intermediate | |
| Kinase domain | Sensitive | Sensitive | Sensitive | |
| Kinase domain | Intermediate | Intermediate | Sensitive | |
| A-loop | Sensitive | Sensitive | Sensitive | |
| A-loop | Sensitive | Sensitive | Sensitive | |
| A-loop | Intermediate | Sensitive | Sensitive |
3.6. Clonal Evolution
The presence of additional chromosomal abnormalities besides Ph chromosome is defined as clonal evolution and is considered to be a feature of advanced CML (55). The most frequent chromosomal abnormalities include, in order, an additional Ph chromosome (38%), trisomy 8 (34%), and isochromosome 17q (56); which have been associated with BCR-ABL1 overexpression, MYC overexpression, and loss of 17p, respectively (57, 58). Other aberrations, such as trisomy 19, trisomy 21, trisomy 17, and deletion of 7, have been reported in less than 10% of patients with clonal evolution. It has been reported that CML-CP patients harbor 0.47 copy number alterations per case (range 0 - 8), but CML-BP patients carry 7.8 copy number alterations (range 0 - 28), supporting the notion that multiple genomic alterations accumulate during CML progression to BP phase (59).
Clonal evolution is associated with reduced response to imatinib, increase in relapse of disease, and reduction in overall survival (60, 61). It is proposed that clonal evolution reflects the genomic instability of the highly proliferative CML progenitor cells associated with disease progression. The frequency of clonal evolution increases with advancing stage, from 30% in accelerated phase to 80% in blast phase (55).
3.7. CML Stem Cells
CML stem cells originate from hematopoietic stem cells (HSCs) by acquiring the BCR-ABL1 mutation. This fusion oncoprotein can transform HSCs, but is not sufficient to transform committed myeloid progenitors which lack inherent self-renewal capability (62). CML stem cells are CD34+/ CD38- cells which have entered the G0 phase of the cell cycle and are therefore quiescent. These cells account for less than 1% of CD34+ cells present at diagnosis (15). It is postulated that this quiescent fraction sustains the disease with constant potential to aggravation. During transition from chronic phase to blast phase, CML stem cells acquire further genetic and/or epigenetic aberrations that provide survival advantage and resistance to programmed cell death.
The resistance of quiescent stem cells to TKI seems to be multifactorial and include altered drug influx and efflux (decreased expression of OCT1 and increased expression of ABCB1 and ABCG2) (63), increased BCR-ABL1 transcript level and decreased degradation of BCR-ABL1 transcript (15). CrkL phosphorylation is a downstream target of BCR-ABL1 and dasatinib, contrary to imatinib, can inhibit its phosphorylation in CD34+ CD38- cells. Dasatinib can also inhibit an earlier progenitor population, but is unable to eradicate the most primitive quiescent stem cells (15, 64). Nilotinib is also ineffective in inhibiting quiescent CML stem cells (65).
4. Conclusions
Targeted molecular therapy has offered excellent clinical responses in the majority of CML patients. The emphasis is currently on overcoming imatinib resistance and the development of alternative TKI engineered to surmount this problem. Some studies have shown the benefit of combination therapy over monotherapy. Combination of TKIs and different targeting agents, including those targeting self-renewal and those inducing apoptosis increases the efficacy of TKIs on CML cells. A better understanding of the mechanisms that underlie TKI resistance, progression to BP, genomic instability and stem cell quiescence is essential to develop curative strategies for patients with CML.