1. Context
Conventional therapeutic agents have distributed non-specifically in body, affecting both target and normal cells. This phenomenon has reduced the efficient dose of the drugs reaching the target cells, then caused suboptimal results due to excessive toxicity (1).
Drug carriers were able to diminish toxicity of the therapeutic agents in neighbor cells and have selectively augmented the amount of drugs accumulated within target cells (2, 3). Although the beneficial effects of carriers have been increasing, some obstacles exist in their implementation for therapeutic purposes. These obstacles include: instability in blood circulation, undesirable bio-distribution, their own toxicity and lack of oral bioavailability (4).
2. Evidence Acquisition
In this review, we have decided to take a glance at the following subjects; first, the types and characteristics of carriers; second, how carriers should develop to improve their therapeutic efficacy?
3. Results
3.1. Delivery Vehicles
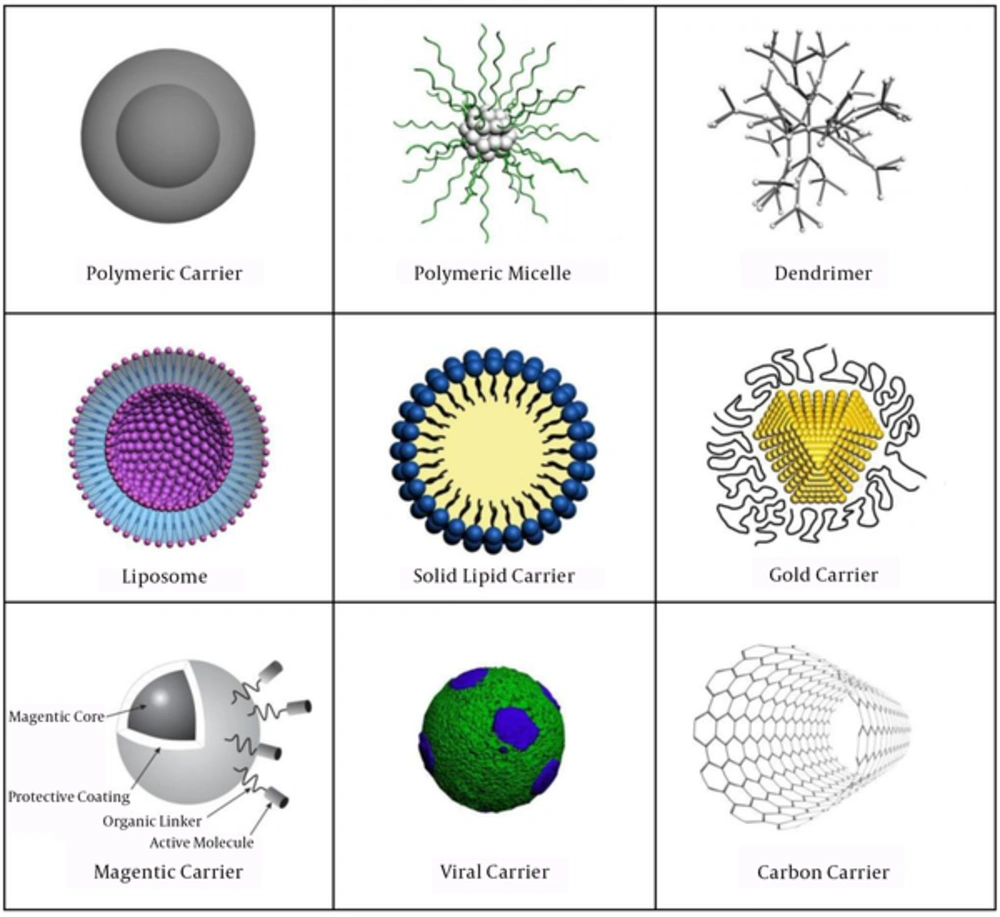
Carriers have used in drug delivery systems have consisted of the simplest form of structures with sizes in the nm or µm ranges, made of polymers (polymeric carriers, micelles or dendrimers), lipids (liposomes), solid lipid carriers, gold carriers, viruses, nanotubes and magnetic carriers (1).
3.1. Polymeric Carriers
Polymeric carriers have composed of natural or synthetic polymers (Figure 1). Depending on the methods used for preparation of polymeric carriers, drugs could be entrapped within the polymer or attached to the carrier matrix leading to the formation of nano/microcapsules and nano/microspheres, respectively (5). In our study, we have reported the synthesis of 166Ho-Poly lactic acid microspheres with solvent evaporation technique (6).
3.2. Polymeric Micelles
Micelles have made of amphiphilic block copolymers (Figure 1). The hydrophobic region has served as a reservoir for poorly water soluble drugs, whereas the hydrophilic region has stabilized the hydrophobic core, and then made the conjugate water soluble (7).
Polymeric micelles, due to their ability in loading of lipophilic molecules into the hydrophobic core, have used to solubilize and deliver poorly water-soluble drugs (8).
Up to now, seven clinical trials using polymeric micelle anticancer drug-targeting systems have done in clinical studies in different phases. Tumor targeting, solubilization of water-insoluble drugs and circumvention of multi-drug resistance (MDR) were the objective of these trials with various combination of drugs (9, 10).
3.3. Dendrimers
Dendrimers have highly branched macromolecules, composed of several extremely branched monomers that emerge radially from the central core (Figure 1). The modifiable surface characteristics of dendrimers enable them to be simultaneously conjugated with several molecules such as imaging contrast agents, ligands or therapeutic drugs, and hence have created a multifunctional drug delivery system (11).
Due to the increasing use of dendrimers in biomedical applications, the biological properties of dendrimers were important. Cationic dendrimers were usually hemolytic and cytotoxic. Their toxicity was generation-dependent and has increased with the number of the surface groups (12). There were indications that cationic dendrimers have cleared rapidly from the blood stream upon the intravenous or intrapritoneal injection. Anionic dendrimers had longer circulation time. Their clearance rate was generation-dependent, and lower generation would have longer circulation time (13). Note that the anionic dendrimers containing carboxylate group were not cytotoxic in the board range of concentrations (14).
Flexibility in the dendrimer families was due to the fact that the surface, internal cavity and dendrimer core could change for different applications. Most of the applications of the dendrimers have based on their multifunctional structure and presence of internal cavities. These properties make the dendrimers suitable for diverse technological applications such as biomedical and industrial applications (15). Such applications were: in vitro diagnostics (15), as a contrast agent in MRI (16), delivery of drugs (17), in boron neutron capture therapy (BNCT) (18), as a vector in gene therapy (19), and in photodynamic therapy (PDT) (20).
3.4. Liposomes
Liposomes have known as spherical lipid vesicles that could be formed via the accumulation of lipids interacting with each other in an energetically favorable manner (Figure 1). The liposome bilayer could be formed from synthetic or natural phospholipids. The important physical and chemical properties of a liposome, including permeability, charge density and steric hindrance, have based on its constituent phospholipids (21).
The use of liposomes as a vehicle in drug delivery systems had several advantages including diverse range of morphologies, compositions, ability to envelope and protect many types of therapeutic biomolecules, lack of immunologic response, low cost and their differential release characteristics (22).
Myocet and Doxil were the fist-approved liposome-based drugs for cancer treatment. Currently, twenty-one liposome-based drugs have been testing by clinical trials (23-25).
3.5. Solid Lipid Carriers
Solid lipid carriers (SLCs), have also referred as lipospheres or solid lipid nanospheres, were solid at human physiological temperature and had a diameter of 50 - 100 nm (Figure 1). The SLCs could be made from diverse range of lipids including mono-, di- and triacylglycerols, fatty acids, waxes and combination of these materials. The SLCs could be formed by replacing oil with solid lipid in an oil and water (O/W) emulsion. Also, the SLCs were biodegradable and biocompatible and could be used in human because of their reduced cytotoxicity (26). These carriers have formed a lipophilic matrix which enabled the drug to load onto it. The important factors have been affecting drugs loading into the SLC matrix were solubility of drug in lipid (drug must be lipophilic), physical and chemical properties of lipid or mixture of lipids, crystalline properties of lipids in biological temperature, and polymorphic form of the lipids. The SLCs have been investigated for delivery of various anticancer drugs with promising results in mouse preclinical phases. Specifically, it has been shown that the SLCs could help to overcome multi-drug resistance problems in cancer therapy (26).
Recently, Kaur and Slavcev have reported the preclinical development of a docetaxel nanocarriers against prostate specific antigen (27).
3.6. Gold Carriers
Gold carriers have consisted of a core of gold atoms (Figure 1) that could be functionalized through the addition of monolayer of thiol (SH)-containing groups (28). Gold carriers could be synthesized in the presence of thiol-containing groups which form a layer around the gold atoms and by considering the stoichiometric ratio of gold to thiol (gold/thiol) utilizing NaBH4 for reduction of AuCl4-salts (29). Research studies have shown that gold carriers were not cytotoxic at cellular levels in a number of human cell lines (30). A recent study has confirmed that PEGylated gold carriers (10 - 30 nm) have not been able to cross from human placenta within 6 hours, and could be used to restrict drug delivery to mother while preventing teratogenic effects on the fetus (31).
The first clinical trial with gold carriers have done in 2009, in which a 27-nm citrate-coated gold carriers (CYT-6091) bound with thiolated PEG and TNF-α have empolyed in patients with solid organ cancers (32).
3.7. Magnetic Carriers
The most unique feature of magnetic carriers (Figure 1) was their reaction to a magnetic force. Magnetic carriers have injected into the bloodstream, and then magnetic fields have focused over the target site (33). The first report of a clinical trial has appeared in 1996 (34), in which noncovalent interactions of epirubicin have used for binding to 100 nm magnetic carriers, and permanent high-energy magnets have used to target tumor tissues. Moreover, magnetic carriers have attracted attentions because of their potential as contrast agents for magnetic resonance imaging (MRI) and heating mediators (hyperthermia) for cancer therapy (35).
3.8. Albumin Carriers
Albumin has been widely employed as a drug carrier. Albumin would be soluble in both water and ethanol. Since albumin has presented in human body, it was non-toxic, and well-tolerated by the immune system. Additionally, albumin due to its higher half-life in blood circulation, had favorable pharmacokinetics and was an interesting drug carrier for passive targeting (36).
Abraxane, the chemotherapy drug paclitaxel has attached to an albumin carrier, was the first drug based on an albumin carrier. Abraxane had advantages over paclitaxel including increased half-life in the circulation and lack of hypersensitivity (37). It has thought that Abraxane has transported into tumor cells by osteonectin or secreted acidic protein rich in cysteine (SPARC) (38).
3.9. Viral Carriers
Viruses naturally and with great efficiency have infected and delivered their genetic contents to host cells (Figure 1). Therefore, viruses have increasingly drawn attractions as favorable carriers for drug delivery. Virus-like particles were genome-free counterparts of viral carriers, and actually could classify as a subclass of viral carriers. Viral carriers, derived from plants and bacteria, were invaluable carriers, because they were not only biocompatible and biodegradable, but also they were non-toxic and non-infectious in humans and other mammals (39). Viral carriers have produced by nature, could regard as one of the advanced drug delivery systems due to their highly symmetrical structure (40, 41). The basic structure of viral carriers could be manipulated in such a way that its internal cavity has filled with drug molecules, imaging reagents, whereas the external surface could be decorated with targeting ligands (42).
3.10. Carbon Carriers
Carbon nanotubes (CNTs) were members of the fullerene structural family composed of benzene rings rolled-up into a tubular structure (Figure 1). CNTs had nanometric dimensions, and they have fallen into two groups based on their structure; single-walled (SWNTs), which consisted of one layer of cylinder graphene and multi-walled (MWNTs) containing multiple concentric graphene layers. The SWNTs had a diameter about 0.4 - 2 nm and length of 20 - 100 nm, while MWNTs were larger in size with a diameter in the range of 1.4 - 100 nm and length from 1 to several µm (43).
The organized structure, ultralight weight, high mechanical strength, high electrical conductivity, high thermal conductivity, methalic or semi-methalic behavior and high surface area (43) were the properties that have caused CNTs to be a suitable carrier in drug delivery system. Combination of these properties made CNT a unique material for diverse applications such as biomedical interventions (44). Increasing interests in use of these properties have made these materials a good candidate for various applications including construction of biosensors for detection of genetic disorders or other molecular abnormalities, substances for cell growth in tissue regeneration, and in drug delivery systems for a broad range of diagnostic and therapeutic agents (43).
Non-functional CNTs were intrinsically hydrophobic. Therefore, the main obstacle in the utilization of CNTs in biology and medicinal chemistry was the lack of solubility in most compatible solvents with biological milieu. To overcome this problem, modification of a CNT surface has mediated by adsorption, electrostatic interaction or covalent bonding of different molecules that render them hydrophilic. These modifications have improved water solubility of the CNTs and their biocompatibility profile. Moreover, due to these modifications, the aggregation of individual tubes through van der Waals forces has reduced (43).
In a recent clinical trial study, Jun Yan reported that CNTs had no toxic side effects on the human body after injection into the tissues around the tumor (45).
3.11. Advantages of Nanoscale Drug Delivery Systems
Biological substances have cleared from the blood stream according to their size. Small particles (1 - 30 nm) were rapidly cleared by the kidney. Carriers having size larger than 30 nm have cleared by reticuloendothelial system (RES), which included macrophages located in the liver and spleen (46). Clearance was also dependent on endothelial fenestral size (46). The size of fenestrae was pretty variable. It was difficult to determine the efficacy and toxicity of drug carriers in different individuals, because age, sex and genetic predisposition affect rate of their clearance (47). Whether nanocarriers would take up by the macrophages or not, depended on opsonization by the innate immune system (48). Surface properties of nanocarriers could affect rate of their clearance by RES. A useful method that has helped large particles escape from opsonization has developed in Rutgers university in the 1960s (49); In a process that called PEGylation, a polymer, polyethylene glycol (PEG; [CH2CH2O]n), has conjugated to a drug carrier.
3.12. Size and Surface Properties of Carriers
In order to be effectively delivered towards the target tissues, carriers should be able to remain in the blood circulation for adequate duration of time. Unmodified conventional carriers have used in drug delivery systems have cleared from blood stream by RES depending on their size and surface characteristics (50). Therefore, the fate of injected carriers could be controlled by modulation of their size as well as surface characteristics.
3.13. Particle Size
Particle size was the most important characteristics of carrier systems. It has determined the in vivo distribution, biological fate, toxicity and the targeting ability of carrier systems (51). The size of nanoparticles have used in drug delivery systems should be large enough to prevent from leakage into the blood capillaries. On the other hand, it should be small enough to escape from macrophages located in RES (1). In our study, microspheres have synthesized with a diameter of 5 - 10 µm without any leakage to the other organs (6).
3.14. Surface Properties of Carriers
Apart from the size of carriers, the surface properties of carriers were another important factor affecting their half-life and fate in blood stream. Surface hydrophobicity of carriers has determined the opsonization. Surface non-modified carriers have opsonized and cleared by the macrophage of RES. Hence, to prolong the circulation of carriers in vivo and to increase the likelihood of the success in drug targeting by carriers, it was necessary to minimize the opsonization. This was possible with two procedures including surface coating of carriers with hydrophilic polymers such as PEG, and construction of carriers from biodegradable block copolymers with hydrophilic and hydrophobic domains (7, 52).
3.15. Types of Targeted Drug Delivery
As outlined above, targeted drug delivery not only has increased the therapeutic effect of drugs, but also, diminished the cytotoxicity to adjacent cells. For the achievement of such conditions, passive targeting and active targeting, have both used.
3.15.1. Passive Targeting
Passive targeting was the preferential accumulation of therapeutic agents in target tissues according to physicochemical or pharmacological factors of the disease. For example, in cancer treatment, Because of higher metabolic demand, cancer cells were required for neovascularization near the tumor mass to supply the oxygen and nutrients (53). This has resulted in disorganized tumor vessels with numerous pores showing enlarged gap junctions between endothelial cells (53). These unique characteristic of tumor vessels has called enhanced permeability and retention effect, have enabled the macromolecules such as nanocarriers to selectively accumulate in a tumor tissue (3).
Another example of passive targeting was acidic environment of tumor cells. A tumor microenvironment would often be hypoxic; Lack of oxygen has caused the tumor cells use glycolysis pathway to get extra energy leaving the extracellular microenvironment acidic (54). Some sorts of pH-sensitive liposomes have designed so that they are stable in physiologic pH but they have disintegrated, and release the drug into targeted tissues that have pH less than physiologic one, such as microenvironment of tumor cells (55).
3.15.2. Active Targeting
Passive targeting with carriers, however, encountered multiple obstacles included: mucosal barriers, nonspecific uptake of the particle and non-specific delivery of the drug (56). Attachment of a ligand or antibody to the carriers, active targeting, was an approach suggested to diminish these restrictions (2).
4. Conclusions
Thanks to complex cellular network in body, delivery of drug to its specific site has been a difficult task. On the basis of the evidence summarized in this review, targeted drug delivery has been coming forward as one of the advanced technique in the field of nanomedicine in the diagnosis and treatment of diseases. Advancement in the field of nanomedicine has been increasing, such that multifunctional carriers that allowed concurrent imaging and therapy have been developed.