1. Background
Cancer is a disease with an infiltrative and destructive nature that has the potential to spread to various organs from its site of origin. This disease is the second leading cause of death worldwide after cardiovascular diseases with 585,720 deaths that have been reported in the United States in 2014 (1, 2). Aggressive treatments such as surgical resection, chemotherapy and radiotherapy are among the usual methods for controlling cancer growth and are used either alone or in combination for treating different stages of malignancies (3). Among these, chemotherapy is one of the most aggressive treatments frequently not tolerated by the majority of patients due to its complications and systemic side effects. Therefore, research that seeks to find effective cures with the least possible side effects is of interest for scientists.
The safe, nontoxic origin of herbs and plants has led to studies of their antitumor and antiproliferative effects (4-8). Throughout history, plants have been valuable sources for novel anti-cancer drugs. Catharanthus roseus and Taxus brevifolia Nutt with their vinca alkaloids (9) and taxol (10), respectively, have led to important advances in anti-cancer treatment. Currently, there are a number of other plants that have the potential for anti-cancer treatments. One approach for studying these plants involves screening the crude plant extracts against different tumor cell lines. In various studies crude extracts of medicinal plants have shown potential anti-cancer properties. A methanolic extract from Teucrium persicum (Lamiaceae) has been shown to potentially inhibit the growth of a highly invasive prostate cancer cell line, PC-3. This extract has also decreased the viability of SW480 colon and T47D breast cancer cells (11). The potent antioxidant and anti-cancer activities of ethanol extract of Alpinia oxyphylla fruits, a traditional Chinese herbal medicine, on six human cancer cell lines (breast, cervix, lung, liver, colon and gastric) was determined by the sulforhodamine B assay (12). Anti-cancer effect of the 50% ethanol-water crude leaf extract of Polyalthia evecta against the HepG2 hepatoma cell line (13), the crude alkaloid extract of Rhazya stricta against A549 lung cancer cells (14) and the extract from young fruit of Longkong (Lansium domesticum) against different cell lines (15) have also been reported.
Worldwide, Iran is among the most geographically diverse countries. The presence of various climatic conditions and ecological factors provide an environment in which numerous varieties of plants grow in different regions of the country. Over 7,000 species of plants grow in Iran among which 1000 are estimated to have medicinal effects (16). According to Iranian folk medicine, many of these plants are used to prevent or treat various diseases such as infections, cancers, and inflammatory disorders. However, the majority have not been investigated for the presence of any biological activities.
2. Objectives
As a continuing effort to screen native Iranian plants for their antitumor effects, the present study aims to investigate the anti-cancer activities of crude extracts of several plant species that grow in Fars Province, Southern Iran. Although a number of these plants are used in traditional medicine as cancer treatments, some have not been investigated for their anti-neoplastic activity against selected tumor cells (17-20).
We investigated the effects of Arctium lappa and Cichorium intybus (Asteraceae), Glycyrrhiza glabra and Alhagi psuedalhahi (Fabaceae), Mentha longifolia, Thymus daenensis, Thymus vulgaris, Satureja bachtiarica, Satureja hortensis (Lamiaceae), and Rheum ribes (Polygonaceae) on five tumor cell lines derived from different cell origins.
3. Materials and Methods
3.1. Preparation of Plants Extracts
The plants were collected from Fars province, Iran and botanically authenticated by specialists. Areal sections of these plants were used for preparation of the methanolic extracts and the voucher specimens were deposited in the herbarium. The herbal materials were washed three times with water, dried in the shade, powdered and macerated in methanol at room temperature for 48 hours. The methanol extracts were then concentrated under reduced pressure using a rotary evaporator. Samples were dissolved in dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO, USA) and then diluted in RPMI 1640 medium at concentration of 2 mg/mL.
3.2. Cell Lines and Cell Cultures
Cell lines used in this study included the human leukemia cell lines, K562 (myelogenous leukemia), Jurkat (T cell leukemia), and Raji (Burkitt’s lymphoma) in addition to the solid tumor cell lines, Fen (bladder carcinoma) and HeLa (cervical epitheloid carcinoma). These cell lines were cultured in RPMI 1640 medium and supplemented with 10% fetal calf serum (Gibco, Berlin, Germany), 100 U penicillin/ml and 100 µg streptomycin/ml. Cells were maintained at 37°C with 5% CO2 and 95% humidity. The cells were fed until confluent and expanded by trypsinization (for adherent cells), then, sub-cultured at lower numbers in new culture flasks. The viability was checked by trypan blue and if it was more than 95%, the experiments were performed.
3.3. MTT Colorimetric Assay
We used the MTT [3-(4, 5-dimethylthiazoyl-2, 5-diphenyltetrazolium bromide)] colorimetric assay to evaluate the cytotoxicity of each extract as previously described (21). In this method a predetermined concentration of tumor cells was first seeded in 96-well plates, treated in triplicate with different concentrations of the extracts (0.1 - 200 µg/mL) and incubated at 37°C with 5% of CO2 and 95% of humidity for 48hours. As a negative control, DMSO (solvent) was added at a concentration equal to the test wells. After the incubation time, we added 10 µL of MTT (5 mg/mL) to each well and the plates were incubated for an additional 4 hours at 37°C. The plates were then centrifuged at 1800 rpm for 10 minutes, the supernatant was removed and 150 µL DMSO was added in order to dissolve the formazan crystals. After 10 minutes of agitation, we read the plates at 570 nm with a reference wavelength of 630 nm in an enzyme linked immunosorbant assay (ELISA) reader. The percentage of inhibition was measured as [1- (optical density of test/optical density of negative control)] × 100. The IC50 value (the concentration of 50% cell inhibition) was calculated from the graph of inhibition percentage against different extract concentrations.
4. Results
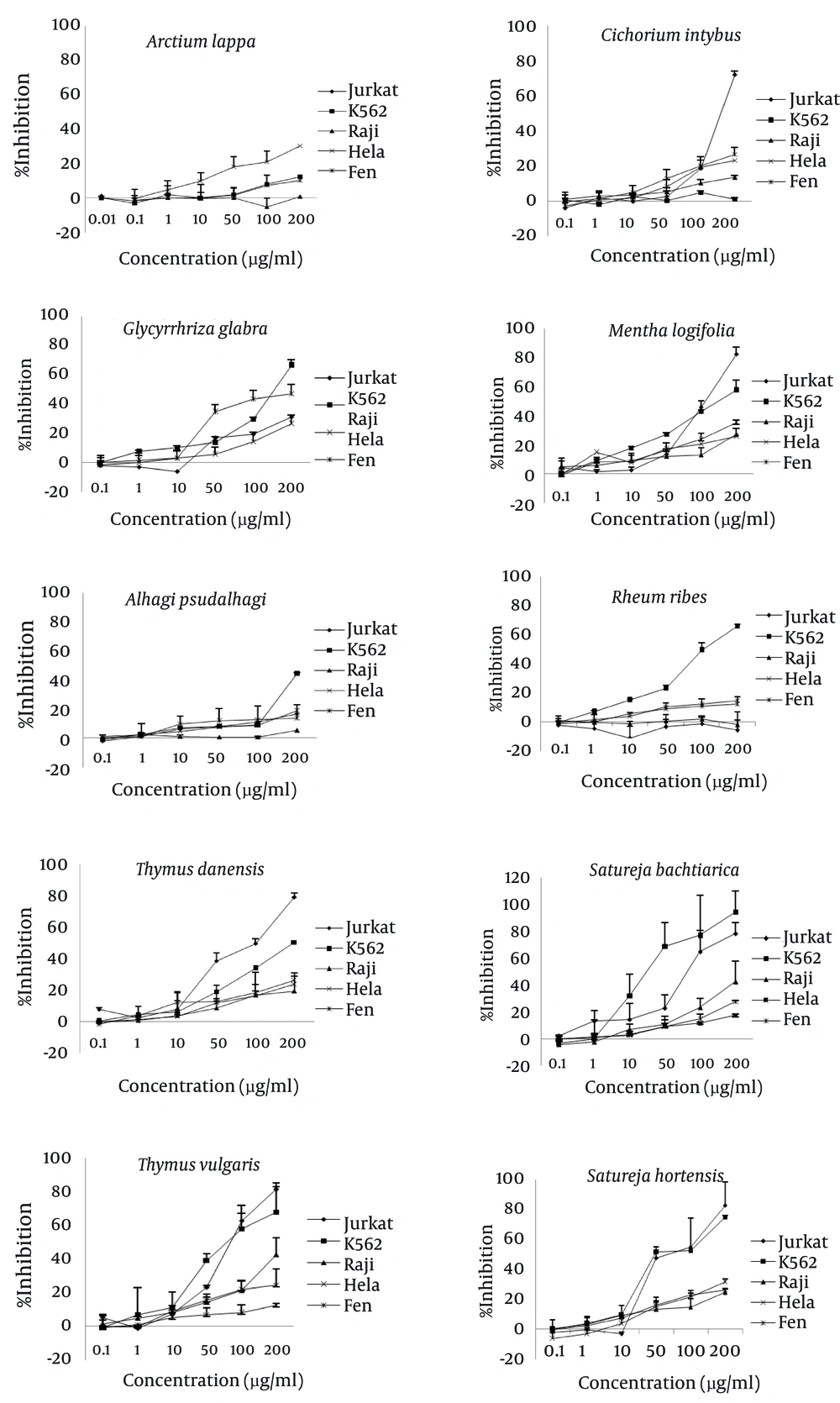
The present study examined the anti-cancer activities of various extracts from plants grown in Iran. We used the MTT colorimetric assay to study in vitro cytotoxic effects of these extracts on different tumor cell lines (Figure 1).
Cells were treated with various concentrations of the extracts for 48 hours. Negative control was cells treated only with solvent (DMSO) at concentrations equal to test wells. Percentage inhibition was calculated according to the formula in the text. Values represent the mean ± standard deviation from three sets of independent experiments.
4.1. Arctium lappa
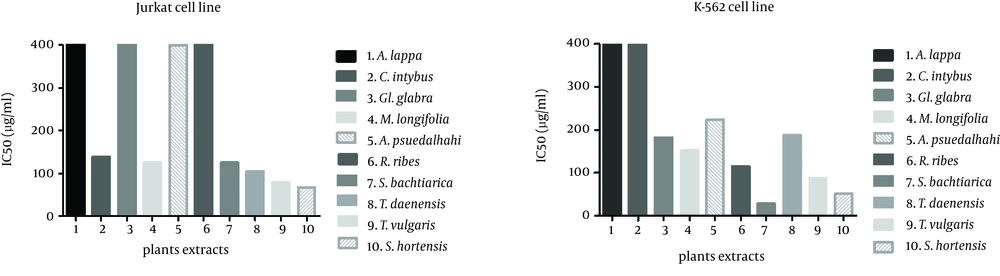
The methanol extract from this plant had its highest inhibitory effect on the HeLa cell line. A total of 30.3 ± 0.2% of cells was inhibited at 200µg/ml of extract concentration. The inhibitory effects on leukemic and Fen cell lines were not as significant as the HeLa cell line. The least inhibitory effect was observed on the Raji cell line which was almost unremarkable. The IC50 of the extract was greater than 400 µg/mL for all cell lines (Figure 2).
Negative control was cells treated only with solvent (DMSO) at concentrations equal to test wells. Percentage inhibition from three sets of independent experiments was determined and then IC50 values were calculated by using curve expert software. IC50 values of plants 1, 3, 5 and 6 on Jurkat cells and plants 1 and 2 on K562 cells are 400 µg/mL or greater.
4.2. Cichorium intybus
The inhibitory effect of this plant’s extract on the Jurkat cell line began at 50 µg/mL of concentration and reached a maximum at 200 µg/mL (72.26 ± 7.7%). The IC50 of the extract for the Jurkat cell line was 138 µg/mL. This extract at a concentration of 200 µg/mL decreased cell growth by 25% in the HeLa and Fen cell lines, but showed no inhibitory effect on K562 cells.
4.3. Glycyrrhiza glabra
Methanol extract from this plant inhibited 66.4 ± 2.6% of K562 cells at a concentration of 200 µg/mL. The resultant IC50 was 169 µg/mL. The Fen cell line showed an IC50 of 208 µg/mL and 47.4 ± 5.3% growth inhibition at 200 µg/mL. At this concentration, the extract had less than 32% growth inhibitory effects on the Jurkat and HeLa cell lines.
4.4. Mentha longifolia
This extract had the highest inhibitory effects on the leukemic cell lines, Jurkat and K562. At 200 µg/ml, the extract inhibited 82.4 ± 4.9% of Jurkat cells and 57.5 ± 6.1% of K562 cells. The IC50 of the extract was 126 µg/mL for the Jurkat and 151µg/mL for the K562 cell lines. The extract inhibited 35.2 ± 2.3% of Fen cells at 200 µg/mL. The effects on other cell lines were weaker with an approximately 27% inhibition at the same concentration.
4.5. Alhagi pseudalhagi
With the exception of the K562 cell line, this extract showed no significant effects on the different cell lines. We observed the highest percentage of inhibition (44.2 ± 0.3%) at 200 µg/mL. The IC50 was calculated as 224 µg/mL.
4.6. Rheum ribes
This extract decreased K562 cell growth to approximately 23% at the 50 µg/mL concentration and up to 66.1±1.2% at the 200µg/ml concentration. The IC50 value obtained for this cell line was 115 µg/mL. The highest concentration of this extract had less than 15% of growth inhibition in the HeLa and Fen cell lines. There was no inhibitory effect on the Raji and Jurkat cell lines.
4.7. Thymus daenensis
This plant had a noticeable effect on the Jurkat cell line where 79.04 ± 4.2% of cells were inhibited by 200µg/ml of the extract. The resultant IC50 was 105 µg/mL. This extract inhibited the K562 cell line with an IC50of 187µg/ml. The extract showed mild growth inhibition on the other cell lines. In the presence of 200 µg/ml of the extract, the Fen cell line was inhibited by 26.1 ± 1.1%, HeLa by 24 ± 6.8%, and Raji by 19.4 ± 9.2%.
4.8. Thymus vulgaris
This extract showed noticeable inhibitory effects on the Jurkat and K562 cell lines. We observed this effect on the Jurkat cell line at a low concentration, which increased in such manner that 50% of cells were inhibited at 79 µg/mL of extract. The highest degree of inhibition, 81.5 ± 1.8%, occurred at 200 µg/mL. The IC50 of this extract on the K562 cell line was 87 µg/mL. A total of 68.1 ± 17% of K562 cells were inhibited at 200µg/ml of the extract, as was 42.6 ± 11% of Raji cells, 29.7 ± 10% of HeLa cells and 12.7 ± 1.1% of Fen cells.
4.9. Satureja bachtiarica
We observed a strong inhibitory effect on the K562 cell line. At a concentration of 28 µg/mL there was 50% inhibition of cell growth which increased to approximately 94% at 200 µg/mL of the extract. In addition, the effect of this extract on the Jurkat cell line was remarkable. In this cell line, the IC50 was 125 µg/ml and 78.7 ± 12% of cells were inhibited at 200 µg/mL. There was less effect of the extract on the other cell lines. The percentage of inhibition at 200 µg/mL on the Raji cells was 42.6 ± 15.6%, whereas for Fen cells it was 27.6 ± 1.3%, and for HeLa cells it was 17.4 ± 1.6%.
4.10. Satureja hortensis
This extract highly inhibited the K562 cell line. We observed the inhibitory effect at 10 µg/mL, which peaked with 75.1 ± 1.3% inhibition at 200 µg/mL of extract. The IC50 was calculated as 52 µg/mL. The IC50 for the Jurkat cell line was 66.7 µg/mL and these cells were 82.8 ± 15% inhibited at 200 µg/mL. At the same concentration other cell lines showed less than 31% growth inhibition.
5. Discussion
We studied the in vitro cytotoxic and anti-cancer activities of methanol extracts of ten plant species that commonly grow in Fars Province, Iran. Some of these plants have been traditionally used worldwide for different purposes. We selected these plants according to their traditional medical use as treatments for various diseases such as infections, tumors and inflammatory conditions (22). The antimicrobial and anti-oxidant properties of the majority of these species have been previously evaluated, which suggested the possibility of anti-proliferative potential for these plants (23-26). Although the cytotoxic effects of some of these plants on prostate, lung, leukemia, and breast cancer cell lines have been evaluated (18-21, 27-30), there is limited cytotoxic data for the majority of these plants and significant information on their anti-tumor properties is not available. We have performed in vitro screening of these plants for their cytotoxic and anti-tumor activities by using the MTT colorimetric assay. The introduction of effective extracts in this screening can open the door for further investigations of mechanisms of action (including cell cycle arrest and induced apoptosis) and the possibility of in vivo testing which may facilitate the way for administration of traditional medicine as cancer treatment (31).
The results showed that Satureja bachtiarica, Satureja hortensis, Thymus vulgaris, Thymus daenensis and Mentha lonigfolia had the highest inhibitory effects on the Jurkat cell line with more than 80% inhibition at a concentration of 200 µg/mL. The same plants strongly inhibited the K562 cell line with more than 50% inhibition at the same concentration. Among the studied plants, Satureja bachtiarica (IC50: 28.3 µg/mL) and Satureja hortensis (IC50: 66.7 µg/mL) had the strongest inhibitory effects on the K562 and Jurkat leukemia cell lines, respectively. To the best of our knowledge, no previous study has researched the anti-tumor effects of Satureja bachtiarica and Satureja hortensis.
Leukemia is a malignancy that results from neoplastic transformation of hematopoietic stem cells or progenitors with aberrant differentiation and proliferation (32). Although there are improvements in treatment success, a notable number of patients with leukemia relapse (33). Although in recent years there have been various practice-changing developments in leukemia treatment, the search for a magic elixir is of interest. Prescription of traditional plant derivatives as medications for leukemia treatment is both noteworthy and affordable. These plants are harmless, safer and more cost effective than present chemotherapeutic agents due to their natural sources.
With respect to the other studied extracts, Cichorium intybus had a notable effect on the Jurkat cell line, although its effect was weaker than the above mentioned plants. Although Rheum ribes (IC50: 115 µg/mL) and Alhagi pseudalhagi (IC50: 223 µg/mL) exhibited remarkable effects on K562 cells, the effects of these plants on other cell lines was not noteworthy. A previous study on Cichorium intybus examined four human cancer cell lines - breast cancer (MCF-7), prostate cancer (LNCaP), amelanotic melanoma (C32) and renal adenocarcinoma (ACHN) by using the sulforodamine B assay. The best response was achieved on melanoma cells with almost 30% of inhibition at a concentration of 100 µg/mL (29). We have not located any published data about the vulnerability of leukemic cell lines to the extracts of this plant.
In the current study, the Raji cell line which originated from Burkett’s lymphoma, was mostly inhibited by Satureja bachtiarica and Thymus vulgaris with nearly 40% inhibition at 200 µg/mL of the extracts. These results supported the power of these two extracts to inhibit leukemia cells and showed reduced sensitivity of this cell line compared to other leukemia cells to these extracts.
Among the studied plant extracts we observed that on solid tumor cell lines only Glycyrrihza glabra had an effect on Fen cells. These cancerous epithelial cells which originated from bladder carcinoma were 50% inhibited by 182 µg/mL of the Glycyrrihza glabra extract. Glycirrhiza glabra is the most well-known plant in the field of cancer research among the studied plants. Several studies on cytotoxic effects of different parts of this plant’s extract have been published. The best effect was seen in prostate cancer as a solid tumor (17). In the current study, this extract noticeably impacted the K562 leukemia cell line; at 200 µg/mL it inhibited approximately 66% of cell growth.
The HeLa cell line which originated from human cervical cancer was relatively resistant in this study as none of the extracts at the highest concentration used had the capability to inhibit 50% of the cells. However, Satureja hortensis with a 31.6% growth inhibitory effect at 200 µg/mL showed the most effect on this cell line. Alhagi pseudalhagi extract despite its very weak effect on other cell lines had approximately 30% inhibition on HeLa cells.
Taken together, the results of this study indicated that Jurkat and K562 leukemia cells were more sensitive to the effects of the studied extracts compared to other cell lines, particularly those which originated from solid tumors. Plants from the Lamiaceae family including Satureja bachtiarica, Satureja hortensis,Thymus vulgaris, Thymus daenensis and Mentha lonigfolia with their effects on both the Jurkat and K562 cell lines showed noteworthy effects. We have proposed that these plants be the subject of more studies in regard to their anti-leukemic effects. Less inhibition was observed on the Raji cell line compared to the above leukemic cells though extracts of Satureja bachtiarica and Thymus vulgaris were mostly effective. The influence of the extracts on solid tumor cell lines was not very strong. Fen cells were mostly affected by Glycyrrihza glabra and HeLa cells by Satureja hortensis which suggested these extracts should be researched further in other solid tumor cell lines in order to discern the underlying mechanisms and responsible compounds.