1. Introduction
Breast cancer is the most common cancer in women and, after lung cancer, is the second leading cause of death in people with cancer. Breast cancer constitutes 32% of the total cancers in women (1). Its prevalence in Iran has been reported as 17.44 per 100,000 people (2-4). Cytochrome P450s are of a specific family of heme-thiol containing proteins. They are classified into 4 groups, 13 families and 9 subfamilies (5). Their most important characteristics are their protective role (6). Cytochrome P450s are involved in the metabolism of medications, exogenous and endogenous compounds, biosynthesis of steroidal hormones and oxidation of fatty acids (6-8). CYP1B1, one of the main enzymes in this family, is situated on chromosome 2p21-22. This gene consists of three exons and two introns, but only its two exons are encoded (8). The CYP1B1 gene may have an important role in tumourigenesis. This gene is highly polymorphic and interpersonal differences resulting from this genetic variation and its various levels of expression may affect the susceptibility of an individual to developing breast cancer (9, 10). In addition, it has been reported that the over-expression of this gene in breast tumours (11) and other tissues such as lung, skin, ovarian and testis (11-13) as well as some intact extra hepatic tissue include breast, uterus, prostate and lung (9, 14).
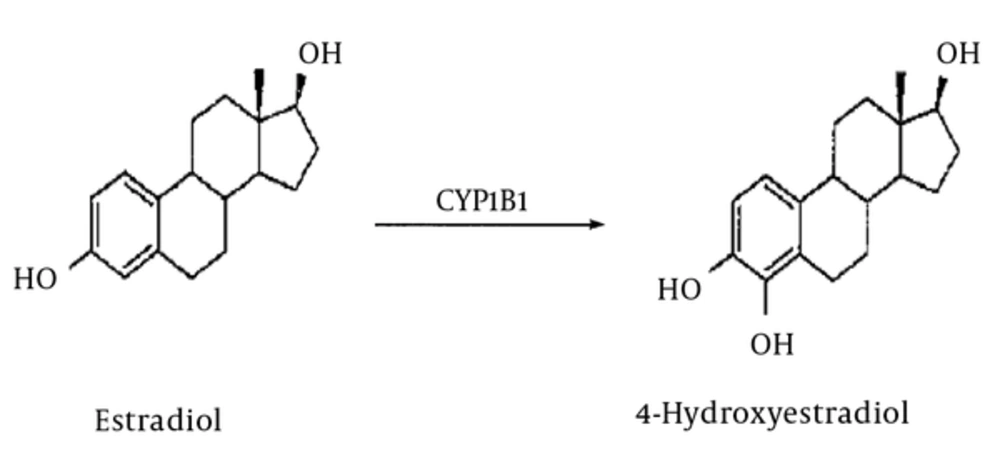
The role of this enzyme is important not only in the activation of several environmental carcinogens such as PAHs and AAs but also in the production of active metabolites that can harm DNA. Activated carcinogens result in the breakage of single-strand DNA and thus increase the number of genetic mutations. The development of the tumour is also enhanced by the reduced effectiveness of anti-carcinogen medications, which are metabolized specifically by CYP1B1 (6, 7, 15, 16). The other role of CYP1B is hydroxylation of estradiol to 4-hydroxystradiol (a genotoxic compound), which is a trigger factor for tumour formation in mammalian cells as it produces active metabolites (17) (Figure 1).
It Shows the CYP1B1 catalysed aromatic Hydroxylation of estradiol to 4-hydroxyestradiol (8).
Polymorphism at the site of the Ala 119 Ser (CYP1B1*2) codon results in a 2- to 4-fold increase in the activity of the CYP1B1 enzyme compared to the natural enzyme (18). This results in a 2- to 4-fold increase in the hydroxylation of the procarcinogen to carcinogenic compounds (18-20). In light of the role of CYP1B1 in converting potential procarcinogen compounds to carcinogenic ones, and the over-expression of this gene in tumour cells, we decided to investigate its prevalence in Iranian women with breast cancer.
2. Methods
We employed a case-control study design in this work. 79 women with breast cancer and 79 healthy women who did not have any history of medical conditions such as diabetes, hypertension or cardiovascular diseases were selected. The healthy women also did not have any incidence of cancer in their first-degree family.
2.1. DNA Extraction and PCR Reaction Conditions
3 - 5 cc blood samples were obtained from the patients, were mixed with EDTA and then stored at a temperature of -20°C. The extraction of DNA from the blood samples was performed by the method of salt saturation (21). The purity and concentration of the DNA were evaluated using a spectrophotometer. PCR-RELP was used to examine the polymorphism. A fragment with 250 base-pairs (bp) in length was proliferated to either analyse the nucleotide polymorphism of G119T in the exon 2 gene CYP1B1 or to identify the genotype of the participant.
The following primers were used in the PCR reaction (made by Macrogen company) include: Forward primer: 5’-CTCGTTCGCTCGCCTGGCGC 3’ and reverse primer: 5’- GAAGTTGCGCATCATGCTGT 3’.
To proliferate the relevant region of the DNA, a reactive mixture was prepared by adding 1 μL in volume from each primer (10 Pmol), 1 μL of genomic DNA (about 10 ng), 10 μL of commercial master mix (2 ×) (Amplicon company of Denmark) and 10 μL of distilled water to the reaction. The stages of the proliferation cycle were as follows: 5 minutes hold at 95°C, 35 cycles of temperature including 1 minute at 95°C, 30 seconds at 64°C, 40 seconds at 72°C and finally 7 minutes at 72°C. The proliferated fragments were then separated by electrophoresis on a 2% agarose gel and observed under UV light.
2.2. Genotype Determination
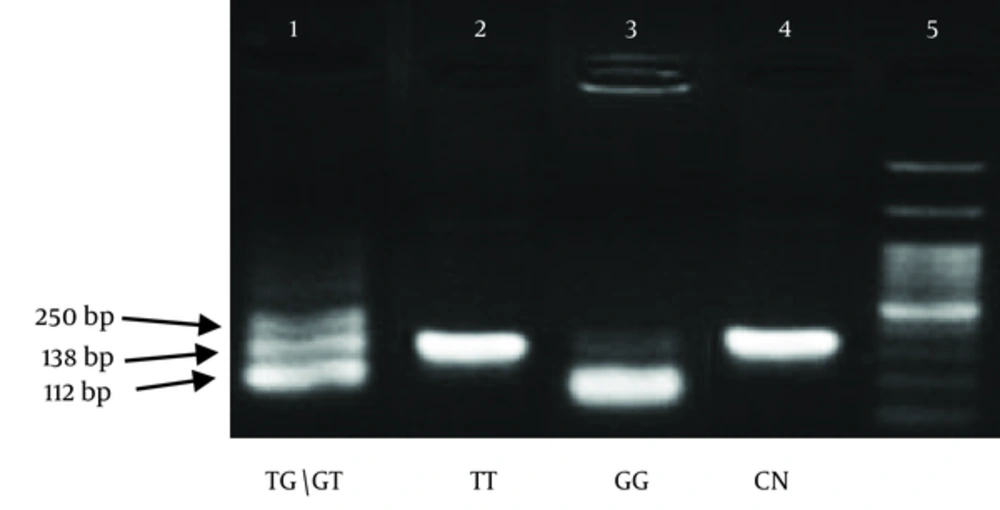
After confirming that the desired fragment had been proliferated, 5 U (0.1 mL) of Pdil enzyme (Thermo company) was added to 5 μL of the PCR products and then incubated at 37°C for 16 - 18 hours. The fragments were then separated by electrophoresis on a 3% agarose gel and observed under UV light. The presence of fragments with 250 bp indicated that the participant had a TT homozygote genotype (without enzymatic digestion); the fragments with 112 and 138 bps indicated that the participant had a GG homozygote genotype and the fragments with 112, 138 and 250 bps indicated that the participant had a GT/TG heterozygote genotype.
2.3. Statistical Analyses
Statistical tests were conducted using the SPSS 19 software package. The x2 test was used to evaluate the difference between genotype distributions in the patient and control groups. The Pearson statistical test was used to examine the correlation between this polymorphism and the risk of developing breast cancer. The odds ratio with a CI: 95% was calculated for estimating the correlation between the polymorphism and the risk of developing breast cancer. In all calculations, probability levels of P < 0.05 were considered to be statistically meaningful.
3. Results
In the patient group, age range was 32 - 70 years with average 49.68 years and in the control group was 30 - 65 years with average 47.34 years. In the patient group the mean weight of the participants was 68 ± 8 kg and 61 ± 2 in the control group. According to the medical records of the patient group, estrogen receptor expression was observed in 58% and progesterone receptor expression in 42% of them.
Of the patient group, 26 (32.91%) had invasive lobular carcinoma, 45 (56.97%) had invasive ductal carcinoma, 5 (6.33%) had ductal carcinoma in situ and 3 (3.79%) had lobular carcinoma in situ. 38 patients had metastatic breast cancer, 16 patients had Grade III breast cancer, 13 patients had Grade II/III breast cancer and 12 patients had Grade II breast cancer.
The frequencies of alleles and homozygote and heterozygote genotypes in this study comply with the rule of Hardy-Weinberg. In the cancer group, it was calculated that there were 42 cases (53.16%) of the TT genotype, 16 cases (20.25%) of the GG genotype and 21 cases (26.59%) of the TG genotype, whereas in the control group, there were 18 (22.79%), 48 (60.76%) and 13 (16.45%) cases of TT, GG and TG genotypes, respectively. The results of some of the PCR experiments are shown in the Figure 2.
The frequency of the G allele in the patient group was 0.34, while in the control group it was 0.69. The frequency of the T allele in the patient and control groups were 0.66 and 0.31 respectively. There was a statistically meaningful correlation between the results of the genotypes between the control and patient groups. The results are summarized in the Table 1.
| Genotyps | Cancer | Control | P Value | Odds Ratio | CI |
|---|---|---|---|---|---|
| TT | 42 (53.16) | 18 (22.79) | 0 | 3.85 | 95% (1.94 - 7.65) |
| TG | 21 (26.59) | 13 (16.45) | 0.223 | 1.84 | 95% (0.85 - 4) |
| GG | 16 (20.25) | 48 (60.76) | 0 | 0.16 | 95% ( 0.08 - 0.33) |
aValues are expressed as No. (%).
4. Discussion
The malignant phenotype appears due to the aggregation of genetic injuries in the normal epithelial cells of the breast tissue. The groups of people who are susceptible to developing breast cancer have various polymorphisms in their genes that are involved in the metabolism of carcinogens and also in repairing the genes in DNA (22, 23). The proteins of cytochrome P460, which have important roles in many metabolic reactions of medications and the synthesis of cholesterol, steroids and lipids, are associated (24) with the risk of developing cancers related to hormones. The hormone oestrogen causes an increase in the probability of mutations in the various divisions of DNA, either by affecting cellular division of genes or by promoting the phase of G1. Generally, steroidal hormones result in cell proliferation by binding to the oestrogenic receptors in the cytoplasm. They therefore affect different genes including growth factors, genes of the growth factor receptor and progesterone receptor, and they can also be considered as a carcinogenic factor (25, 26). Various studies conducted on the CYP1B1 enzyme have indicated that the polymorphisms of this gene can change the enzymatic activity and the catalytic features of the enzymes (27, 28). Considering four polymorphisms in the codon of Arg48Gly (CYP1B1*1), Ala119Ser (CYP1B1*2), Leu432Val (CYP1B1*3) and Asn453Ser (CYP1B1*4), it becomes clear that they have more hydroxylation activity than normal types, and all of them are related to the susceptibility of developing a cancer (27, 29-32).
In this study, we have evaluated the correlation between polymorphisms in CYP1B1 related to changing the Ala119Ser and the risk of developing breast cancer in Iranian women. A relationship between this polymorphism and the progression of several important cancers, including colorectal cancer (7), uterine leiomyoma (33), prostate cancer (34, 35) and breast cancer (27, 32, 36, 37), has been identified. We found that the frequency of the G allele in a group of cancer patients was 0.34, while in a control group of healthy women the frequency of the G allele was 0.69. Statistical analysis showed a meaningful correlation between the patient and control groups in terms of a comparison of their genotypes. There was no statistically meaningful correlation in the results of the frequency of the TT genotype between the two groups. Furthermore, the calculated odds ratio (OR: 3.85 CI 95% (1.94 - 7.65)) indicated that the presence of this genotype in a person would cause them to have a higher risk of developing breast cancer. The calculated odds ratio of the GG genotype (OR: 0.16 CI 95% (0.08 - 0.33)) showed that the presence of this genotype is not indicative of a higher risk of developing cancer. However, there was a statistically meaningful correlation between this genotype and the risk of developing cancer. No meaningful correlations for the TG genotype were observed. Nevertheless, the odds ratio (OR: 1.84 CI 95% (0.85 - 4)) showed that those with this type of genotype do have a heightened risk of developing breast cancer. Our results demonstrated the correlation between polymorphism of CYP1B1 with the risk of developing breast cancer.
Moreover, there was a meaningful correlation observed between polymorphisms of CYP1B1 (such as rs 1056827) and breast cancer in other studies on Chinese, Japanese and Turkish women (38-40). Our results were confirmed when compared with other studies. In studies by Jiao et al (2010) (41), a meta-analysis study by Economopoulos et al. (2010) (42), a study by Reding et al. (2009) (43) and a study by Rylander-Rudqvist et al. on people with breast cancer indicated a significantly meaningful correlation between polymorphisms of the P450 1B1 gene (Ala119Ser) and a risk of developing breast cancer (44). In addition, Hanna et al. (2000) evaluated the effectiveness of the pharmacokinetics of polymorphisms on cytochrome P4501B1 on the activity of hydroxylation of oestrogen, and their results demonstrated that changing the activity of oestrogen hydroxylation by CYP1B1 can increase the risk of developing breast cancer (45). Furthermore, this polymorphism has been related to the risk of developing prostate cancer (35), uterine leiomyoma (46) and primary open-angle glaucoma (47). It appears that the polymorphism is a trigger substance for cancer that affects the efficacy of the CYP1B1 enzyme and plays a key role in the bio-variation of oestrogens and reactive pro-carcinogens with DNA (41).
Some genetic variants in estrogen metabolism pathway may be increased susceptible women. Such as our finding that suggested Ala 119 Ser polymorphism in the hormone metabolism pathways associated with breast cancer.