1. Background
Glial tumors including astrocytomas are the most common primary adult brain tumors. Astrocytomas are a heterogeneous group of tumors with varied clinical behavior. According to world health organization (WHO) grading system, these neoplasms are classified from grade I to IV (1, 2). The WHO grading scheme is based on numerous pathological criteria including mitosis, cell morphology, atypia, endothelial proliferation and tumor necrosis. The prognosis varies dramatically from uncommon grade I (pilocytic astrocytoma) which is curable with surgery alone in most cases to the most common variant, grade IV (glioblastoma) which is usually incurable despite exploiting multi-modality treatments. The Central Brain Tumor Registry of the United States reported overall survival rate at 5 years for pilocytic astrocytom and fibrilary astrocytom around 94% and 48% respectively. In this report, 3-year overall survival has been around 35% for astrocytom anaplastic and 8% for gliobalstoma multiform (3). A retrospective study in the north-east of Iran on 324 patients shows compatible results (4).
Previous trials have investigated the effects of different factors that can influence the prognosis of these patients (1, 2).
2. Objectives
This study aimed to investigate treatment results and prognostic factors in patients with low grade and high grade astrocytoma separately in our centers in Iran.
3. Patients and Methods
This retrospective cohort study was performed on patients with brain astrocytoma who were referred to our oncology departments, between October 2000 and December 2011. The data regarding clinical and pathological characteristic, treatment strategy were extracted. Subjects less than 16 years of age and patients with insufficient data regarding their clinico-pathological characteristics, treatment and follow-up were excluded.
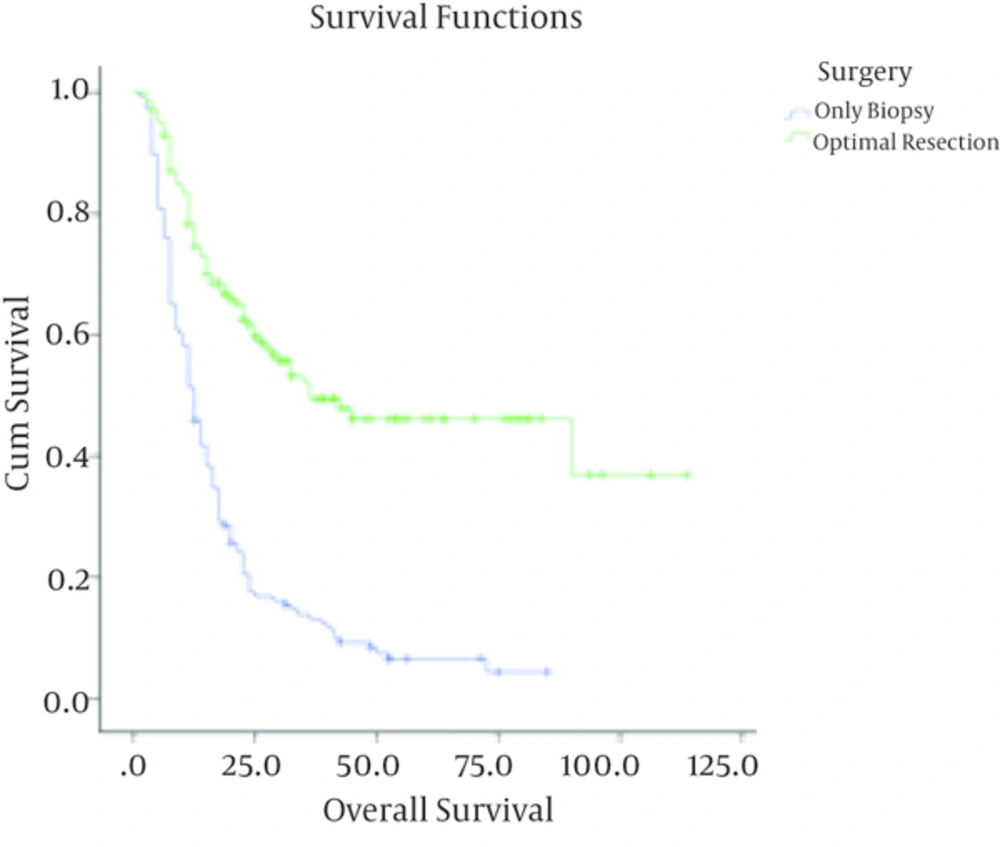
Surgical resection was considered in cases with removing more than 50% of lesion, while residual more than 50% of primary lesion (based on lesion size on imaging) was classified as biopsy. Regarding the performance status, patients were classified into two groups of ambulatory and not ambulatory (having major neurological deficit and/or Karnofski performance status score of less than 60).
3.1. Statistical Analysis
Descriptive statistical analysis, tables and graphs were prepared. Kaplan-Meier model was used to determine the survival rate. Log-rank test were utilized to compare survival curves between groups. Multivariate analysis of prognostic factors was performed by Cox regression test. Data were analyzed by SPSS version 19.
4. Results
415 patients with astrocytoma with a median age of 43 (range; 16 to 84) and a male to female ratio of 252:163 (1.54) were recorded. The median age for patients with low grade and high grade tumors was 33 (range; 16 - 74) and 50 (range; 16 - 84) respectively. From all cases, 40(9.6%) were grade I, 88 (21%) grade II, 71 (17.1%) grade III and 216 (52%) were grade IV. The tumor locations were as follows: 388 cerebral hemispheres (93.5%), 11 midbrain (2.7%), 3 pineal region (0.7%), 9 cerebellum (2.2%) and 4 brainstem (1%). 131 patients (28%) had a history of seizure. Among patients with high grade and low grade astrocytoma, 284/287 (99%) and 118/128 (92.2%) were supratentorial respectively. 231 cases (55.7%) were non-ambulatory which was significantly more frequent in patients with high grade than those with low grade tumors (60.6% vs. 44.5%, P = 0.002).
Surgical resection had been performed in patients with grade I and II tumors in 24/40 (60%) and 55/88 (62.5 %) of cases respectively. All cases had undergone radiation therapy with a median radiation dose of 54 Gy (50 - 56 Gy). Among patients with high grade astrocytoma, 41/71 (57.7%) with grade III and 99/215 (46%) with grade IV pathologies had undergone surgical resection. From patients with grade III and IV astrocytoma, 67 (94.4%) and 198 (91.7%) could complete their radiation therapy courses with a median dose of 58 Gy (range; 45 to 60 Gy) and 46 (64.8%) and 145 (67.8%) cases had received adjuvant chemotherapy respectively. The most common chemotherapy regimen was Carmustin plus Vincristin (157/197, 79.7%). Concurrent chemoradiation and adjuvant t chemotherapy with Temozolomide was administered in 5 (7%) of patients with grade III and 29 (13.4%) of patients with grade IV tumors
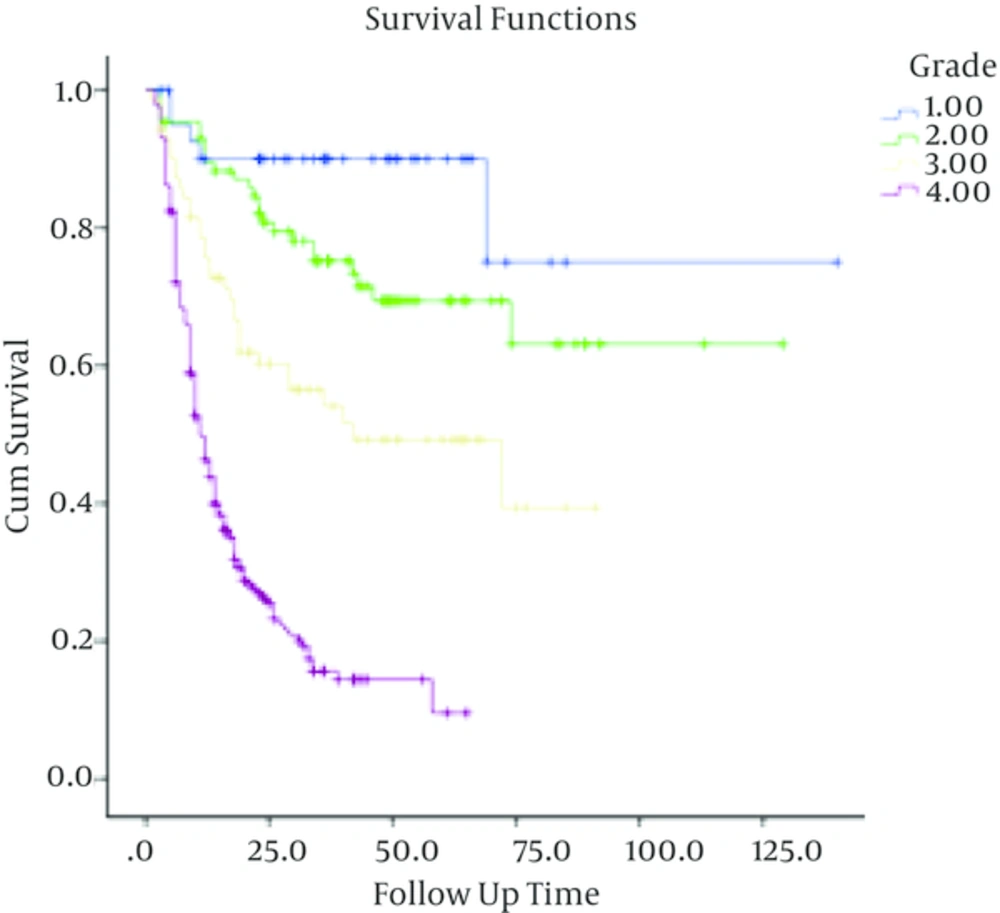
5-year overall survival in astrocytomas with grades I to IV were 90% ± 4.7%, 69% ± 5.5 %, 49.2% ± 6.6% and 14.4% ± 3% respectively.
4.1. Low-Grade Tumors
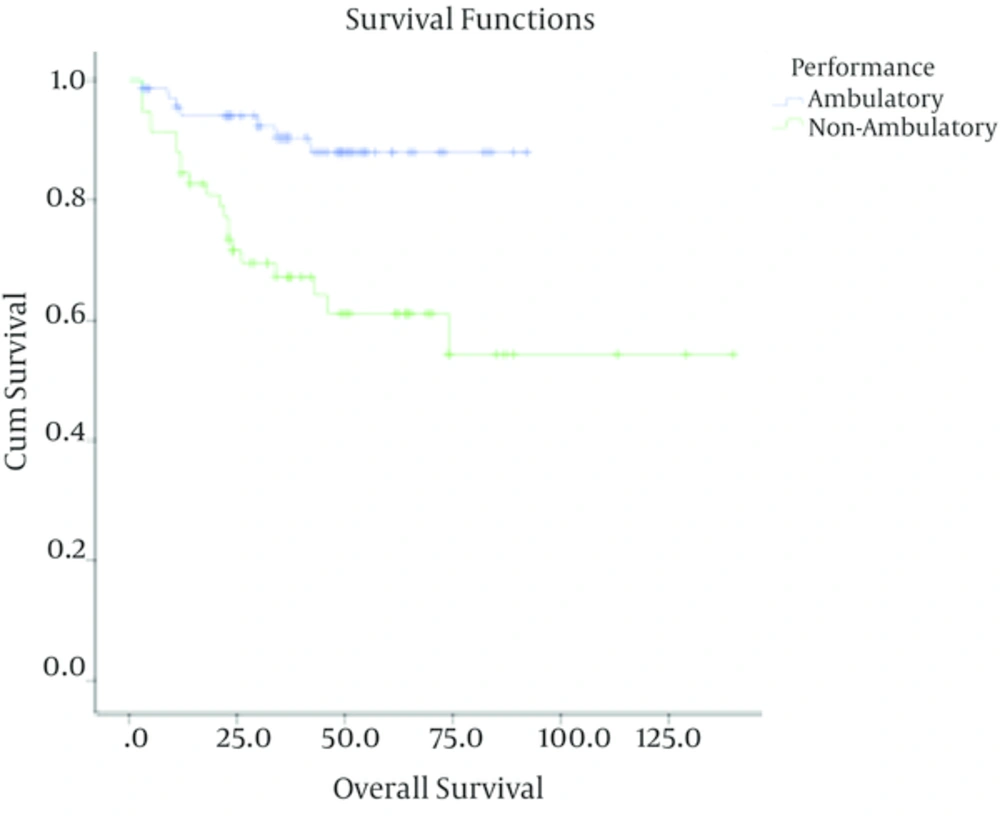
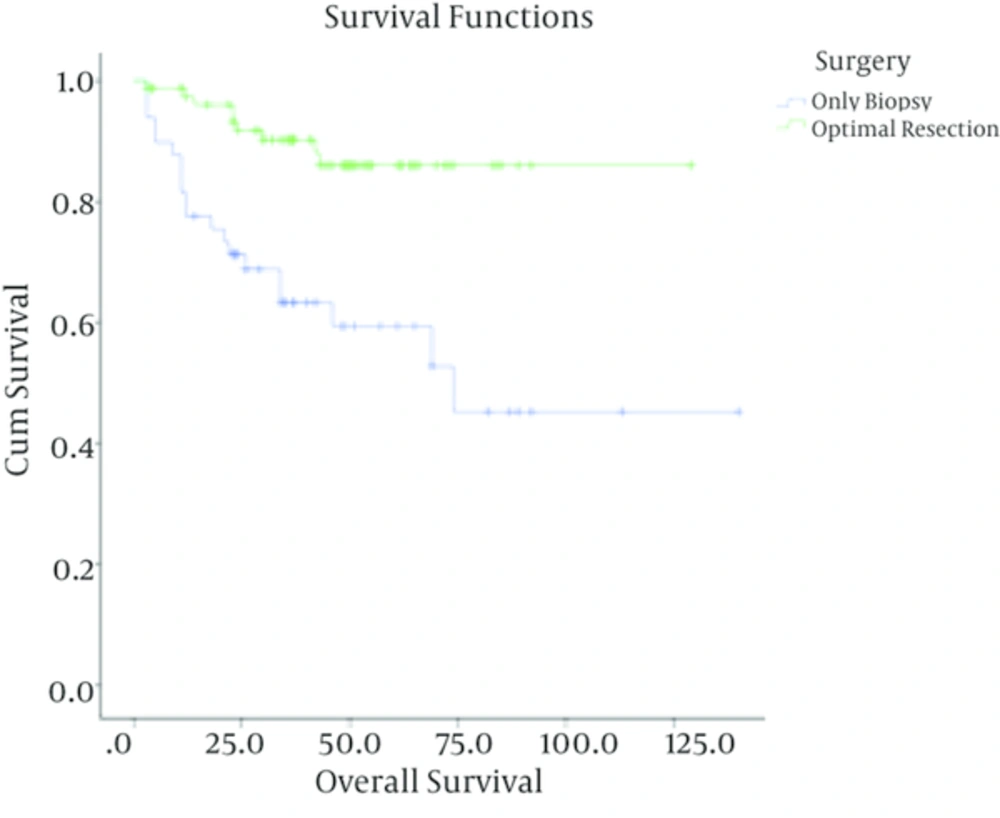
In patients with low-grade astrocytoma, with a median follow up time of 37 months (ranging from 3 to 140 months), the rate of recurrence among grades I and II was 4/40 (10%) and 28/88 (31.8%) respectively with a 5-year survival rate of 90% ± 4.7%, 69% ± 5.5 %. In these cases, tumor grade (I Vs. II), being ambulatory (P < 0.001) and performing optimal surgery (P < 0.001) were correlated with better outcome. Meanwhile, age (P = 0.3) and sex (P = 0.4) were not associated with overall survival. Multivariate analysis showed that tumor grade, performing optimal surgery and performance status were independent prognostic factors in these patients (Table 1).
| Factor | Number | Death, No. (%) | 5-Year Survival, (%) ± SE | Log-Rank, P Value | COX-Regression |
|---|---|---|---|---|---|
| Grade | 0.01 | 0.01 | |||
| I | 40 | 3 (7.5) | 92.1 ± 4 | ||
| II | 88 | 26 (29.5) | 69.1± 5.1 | ||
| Sex | 0.4 | - | |||
| Male | 71 | 14 (19.7) | 79.8 ± 5 | ||
| Female | 57 | 15 (26.3) | 69.9 ± 7 | ||
| Age | 0.6 | ||||
| ≤ 30 | 54 | 11 (20.3) | 79.8± 5.9 | ||
| > 30 | 74 | 18 (24.30) | 72.8 ± 5.8 | ||
| Surgery | < 0.001 | 0.001 | |||
| Optimal resection | 79 | 9 (11.4) | 86.1 ± 7.7 | ||
| Biopsy | 49 | 20 (40.8) | 59.3 ± 4.4 | ||
| Performance | < 0.001 | 0.01 | |||
| Ambulatory | 71 | 8 (11.2) | 88.2 ± 4.3 | ||
| Non-ambulatory | 57 | 21 (36.8) | 60.2 ± 7.2 |
4.2. High-Grade Tumors
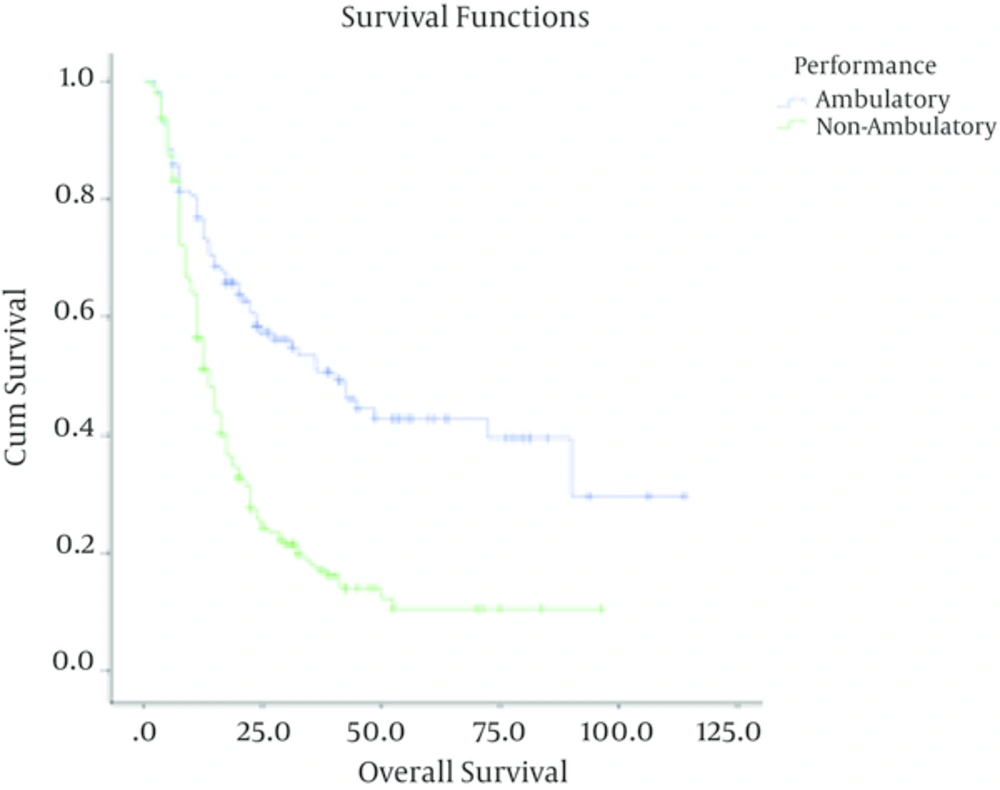
In high grade gliomas, with a median follow up time of 13 months (range; 1 - 91 months), the number of documented recurrences among patients with grade III and IV tumors was 33 (46.5%) and 166 (76.6%) respectively. Grade (grade III Vs. grade IV), age less than 50 (P < 0.001), being ambulatory (P < 0.001), performing optimal surgery (P < 0.001) and receiving chemotherapy (P = 0.02) were significantly associated with higher overall survival. In these patients, sex (P = 0.8) was not correlated with overall survival.
| Factor | Number | Death, No. (%) | 5-Year Survival, (%) ± SE | Log-Rank, P Value | COX-Regression |
|---|---|---|---|---|---|
| Grade | < 0.001 | < 0.001 | |||
| III | 71 | 33 (46.4) | 49.2 ± 6.6 | ||
| IV | 216 | 199 (92.1) | 9.6 ± 4.4 | ||
| Sex | 0.8 | - | |||
| Male | 181 | 124 (68.5) | 24.9 ± 3.7 | ||
| Female | 106 | 75 (70.7) | 18.5 ± 5.1 | ||
| Age | < 0.001 | < 0.001 | |||
| ≤ 50 | 144 | 90 (62.5) | 29.6± 9.7 | ||
| > 50 | 143 | 109 (76.2) | 14.6 ± 3.7 | ||
| Surgery | < 0.001 | < 0.001 | |||
| Optimal resection | 140 | 64 (45.7) | 46.4 ± 5.1 | ||
| Biopsy | 146 | 134 (91.7) | 4.3 ± 2.3 | ||
| Performance | < 0.001 | < 0.001 | |||
| Ambulatory | 113 | 58 (51.3) | 39.4 ± 5.9 | ||
| Non-ambulatory | 174 | 141 (81.1) | 10.5 ± 3.1 |
5. Discussion
In this retrospective study, the most common histological type of astrocytoma in adult cases was glioblastoma astrocytoma. Grade I astrocytoma was found in only 10% of cases. The median age for patients with malignant astrocytoma was higher as compared to cases with low grade tumors. Most cases especially high grade astrocytomas were found in cerebral regions. These results in our region are similar to the previous reports from other nations.
Grade I astrocytoma is very rare in adults. However, we found a favorable outcome in these cases. In patients with low grade astrocytoma the extent of tumor resection and performance status were independent prognostic factors. The importance of surgical resection has been proved in previous studies (5-9). Being non-ambulatory as having major neurological deficit and/or Karnofsky performance status of less than 60% can be a reflection of larger tumors and probably bigger residual tumor following surgery. These factors have been shown to be unfavorable prognostic factors in the previous studies (7, 10, 11).
In a Turkish study by Durmaz et al. (12), 53 patients with low-grade glial tumor were investigated between 1980 and 2006. The efficacy of age, sex, location of tumor, the extent of resection, the presence of seizure, radiotherapy and performance status using Karnofsky score were evaluated on survival rates of patients. There was not found a significant association between tumor location and overall survival (P = 0.65). In this study, survival rate was independently affected by the extent of resection (P = 0.36) (12).
In the study by Durmaz et al. (12), overall survival was not associated with gender (P = 0.19). These findings were consistent with the present results that there was not an association between gender and overall survival (80 ± 5 for men vs. 70 ± 7 for women), (P = 0.04)
In Durmaz et al.’s study (12), the patients were divided into two groups based on Karnofsky performance score: patients who were scored 70 or less and patients who were scored over 70 that the patients with performance scores In Durmaz’s study, the survival values of the patients who were under the age of 40 were significantly better (P = 0.02). The median survival rate for the patients who were over the age of 40 was significantly (108 months) better than that of the patients under the age of 40 (168 months). Patients with performance status of over 70 survived longer than those with scores 70 or less (P = 0.03).
Another Canadian study by Leighton et al. (13) was undertaken on patients with low-grade tumors during 17 years follow-up. The patients who had performance scores higher than 70% survived longer than those with performance scores 70% or below (P < 0.001).
In our series with malignant astrocytoma, patients with glioblastoma had a dire prognosis with a 5-year survival rate around 9%. As compatible with previous studies (14, 15), patients with grade III astrocytoma had much better outcome with a 5-year survival of around 49%.
The extent of surgical resection had also significant effect on survival in patients with high grade astrocytoma (14-20). In a study by Buckner (20), the survival rate for the patients who were treated with biopsy was lower than that of the patients who underwent surgical resection. Patients who were ambulatory had also significantly more favorable outcome. These results were compatible with the results of previous trials (14, 15, 19-21). In the present study, patients with malignant astrocytoma who were younger than 50 had significantly better survival rate than older cases. More favorable results have been also shown in younger patients in previous studies (15, 16, 22).
The effect of adjuvant chemotherapy on survival in patients with high grade astrocytoma has been investigated in multiple trials. In a review article by Stewart investigated the effects of radiotherapy and radiotherapy plus chemotherapy on survival values of patient with high-grade tumors. Survival rate was significantly better in patients who were under chemotherapy plus radiotherapy treatment than patients who were under radiotherapy alone (P < 0.001) (23).
Based on the Cox proportional-hazard analysis, age, receiving chemotherapy, surgery types and performance status were significant predictors of survival.
Performing optimal surgery and good performance status were associated with more favorable survival in both low and high grade astrocytomas. In high grade Astrocytomas, patients younger than 43 and those who received chemotherapy had better overall survival.