1. Background
Gestational trophoblastic disease is a group of heterogeneous conditions; ranging from simple molar pregnancy to gestational trophoblastic neoplasia with aggressive behavior and metastasis (1-3). It has an incidence rate of 1 per 1000 pregnancies in Western countries (4) and is more common among Eastern populations (5, 6). Although the metastatic form could be potentially fatal, early diagnosis and chemotherapy makes it as one of the most curable solid tumors (7-9).
Current guidelines suggest measuring weekly serum βhCG (human chorionic gonadotropin) following the evacuation of pregnancy products and in case of plateau or rising pattern; the persistent gestational trophoblastic disease would be suspected and chemotherapy should be started (10, 11) it is a great advantage if one could predict aggressive behavior of the disease before an increase in serum βhCG. Researchers have studied molecular pathogenesis of gestational trophoblastic neoplasia and some found p53 and c-erbB-2 to have a role in malignant behaviors of these tumors (12-16). However, there were controversies whether p53 and c-erbB-2 expression could act as tumor markers.
2. Objectives
In this survey we planned to study patients with molar pregnancy using immunohistochemistry staining to 1) assess p53 and c-erbB-2 expression in trophoblastic tissue, 2) to study the relationship between their expression intensity and progression of a molar pregnancy to gestational trophoblastic neoplasia, and 3) to determine a cut off value for p53 and c-erbB-2 expression intensity in case of correlation with aggressive behavior of molar pregnancy.
3. Patients and Methods
3.1. Population
In a prospective cross sectional study, we included patients with primary diagnosis of molar pregnancy referring to oncology clinic of Qaem hospital, affiliated to MUMS. All patients underwent evacuation and curettage, followed by weekly βhCG measurements. Patients were divided into two groups: (1) gestational trophoblastic neoplasia (GTN) group if serum βhCG level rose or did not change during study; (2) simple molar pregnancy group whose serum βhCG underwent gradual decrease. Serum βhCG level < 5 mIU/mL was considered as normal. Patients’ specimen of curettage were referred to pathology laboratory of hospital for histological and immunochemistry studies.
3.2. Histological and Immunochemistry Studies
Immunohistochemistry staining was performed on multiple 4 µm sections of paraffin blocks provided from formalin fixed trophoblastic tissues. In order to evaluate the immunoreactivity of c-erbB-2 oncogene and p53 tumor suppressor gene, we applied a polymer based Dako Envision Tm system technique; (Do-7, Dakocytomation, N1581, DAKO Corporation, Carpiteria, CA 93013 USA) for p53 antigen and (Clone PN2A, Dakocytomation, Denmark A/S, DK-2600 Glostrup, Denmark) for c-erbB-2. Normal prostatic tissue and breast cancer slides were used as positive controls for p53 and c-erbB-2 respectively due to company protocols. As negative controls, phosphate buffered saline (PBS) was substituted with antibodies. All slides were observed by a single pathologist under a light microscope (Olympus B × 50; Olympus optical Co, Ltd, Tokyo Japan). The rate of p53 expression was reported as percentage of cytotrophoblastic and syncytiotrophoblastic cells with positive nuclear immunoreactivity. The c-erbB-2 oncogene expression rate was calculated as percentage of cells with positive membranous staining. To grade p53 staining intensity semi quantitatively, we applied 0 for no stained cells, + for staining of less than 10% of cells, ++ for 10 to 50% of cells, +++ for staining in more than 50% of cells. To score c-erbB-2 staining intensity we used negative as no or less than 10% of cells’ membranes stained, 1+ for faint membranous staining in more than 10% of cells, 2+ for weak to moderate complete membranous staining in more than 10% of cells and evaluate 3+ as strong for complete membranous staining in more than 30% of cells (17). All tissue preparation stages were performed based on Dako Envision Tm company protocols (18).
3.3. Statistical Analysis
Data were entered on SPSS for windows software version 21. Categorical data were analyzed by chi-square or exact Fischer test. Mann-Whitney test and independent sample t-test were applied to compare continuous variables. To estimate a cut off for percentage of positive immunostained cells ROC (receiver operating characteristic) curve analysis was applied to evaluate the risk of transformation of molar pregnancy to gestational trophoblastic neoplasia. The P value < 0.05 was considered statistically significant.
3.4. Ethics
Informed consents were signed by all patients. All diagnostic and therapeutic interventions including evacuation of pregnancy products, serial weekly measurement of serum βhCG, and histological evaluation for their primary diagnosis (complete or partial molar pregnancy) were performed according to indications for patients with molar pregnancy diagnosis. Immunohistochemistry expenses were covered by the research budget.
4. Results
We included 58 patients: 30 with final diagnosis of Gestational Trophoblastic Neoplasia (GTN) and 28 with simple molar pregnancy. Although Patients with GTN diagnosis had a higher age average, gravidity and parity number in comparison with simple molar pregnancy group, only age had a significant correlation with GTN. The primary diagnosis of 28 patients with (GTN) was complete molar pregnancy (P value < 0.05). Table 1 displays patients’ demographics.
| Simple Mole | GTN | P Value | |
|---|---|---|---|
| Final diagnosis | 28 | 30 | |
| Primary diagnosis | 0.00 | ||
| Complete mole | 18 | 28 | |
| Partial mole | 10 | 2 | |
| Age, mean ± SD | 26.2 ± 7.4 | 31.9 ± 9.0 | 0.01 |
| Gestational age, mean ± SD | 11.3 ± 4.0 | 11 ± 3.2 | 0.79 |
| Gravity number, mean ± SD | 1.7 ± 1.5 | 3.1 ± 2.3 | |
| Parity number, mean ± SD | 0.5 ± 1.5 | 1.9 ± 2.2 |
Abbreviation: GTN, Gestational Trophoblastic Neoplasia.
aP value < 0.05 considered significant.
The immunohistochemistry staining results in GTN group showed a significantly higher average percentage of cytotrophobalst and syncytiotrophoblast with positive nuclear immunoreactivity for p53 in comparison with simple molar patients. The membranous immunostaining of cytotrophoblast for c-erbB-2 was also significantly greater in GTN group. Patients with primary diagnosis of complete mole had significantly higher percentage of p53 positive cytotrophoblast and syncytiotrophoblast in comparison with partial mole group (Table 2 and Figure 1).
| GTN | Simple Mole | P Value | Complete Mole | Partial Mole | P Value | |
|---|---|---|---|---|---|---|
| P53 Cytotrophobalsts | 41.6 ± 25.4 | 5.3 ± 9.3 | 0.000 | 27.9 ± 26.7 | 9.0 ± 21.1 | 0.007 |
| P53 Syncytiotrophoblasts | 19.3 ± 18.8 | 1.2 ± 1.1 | 0.000 | 12.1 ± 17.1 | 4.0 ± 9.8 | 0.027 |
| C-erbB-2 Cytotrophobalsts | 18.4 ± 26.4 | 2.5 ± 8.6 | 0.000 | 11.7 ± 21.6 | 6.2 ± 20.1 | 0.053 |
| C-erbB-2 Syncytiotrophoblasts | 5.4 ± 14.5 | 1.6 ± 5.4 | 0.508 | 4.0 ± 12.1 | 1.6 ± 5.7 | 0.437 |
Abbreviation: GTN, Gestational Trophoblastic Neoplasia.
aP value < 0.05 considered significant.
bValues are expressed as mean (SD).
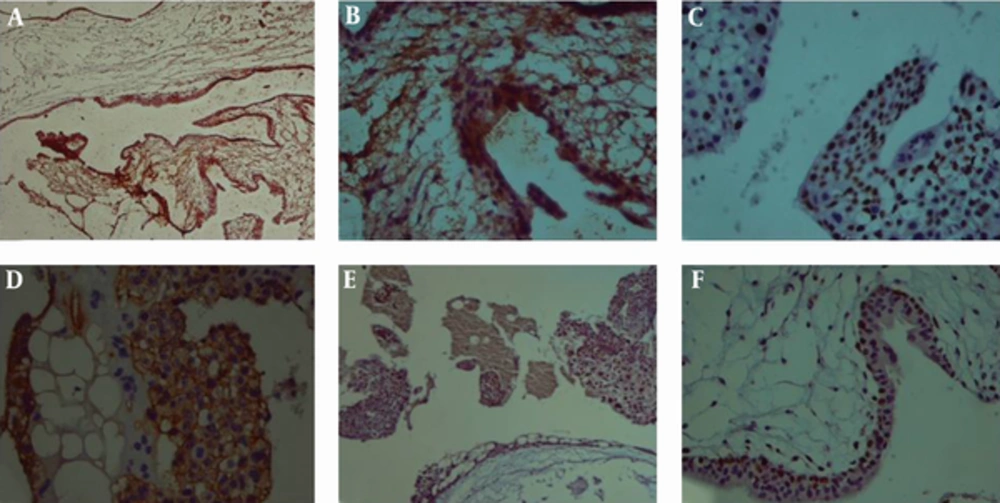
(A), Nuclear cytotrophoblasts and syncytiotrophoblasts immunoreactivity (× 100) (B), Negative immunoreactivity of syncytiotrophoblasts and positive nuclear immunoreactivity of more than 80% of trophoblasts (C), positive cytoplasmic immunoreactivity of trophoblasts for c-erbB-2 with fairly no staining of syncytiotrophoblasts (D), Hydropic villi with trophoblastic vacuolization and positive immunoreactivity in about 80% of trophoblasts (× 40)(E), (× 100) (F).
Patients in GTN group displayed a significantly higher immunoreactivity score for p53 among both cytotrophoblast and syncytiotrophoblast as compared to patients with simple molar pregnancy. Membranous immunoreactivity score of cytotrophoblast for c-erbB-2 marker were also higher among GTN group. Syncytiotrophoblast showed fairly similar immunoreactivity for c-erbB-2 marker in both GTN and simple molar pregnancy groups with no statistical meaningful difference (Table 3).
| P53 Cytotrophoblast | P53 Syncytiotrophoblast | C-erbB-2 Cytotrophoblasts | C-erbB-2 Syncytiotrophoblast | |||||
|---|---|---|---|---|---|---|---|---|
| SMP | GTN | SMP | GTN | SMP | GTN | SMP | GTN | |
| Negative | 1 (3.8) | 1 (3.3) | 6 (23.1) | 2 (6.9) | 23 (88.5) | 7 (23.3) | 23 (88.5) | 25 (83.3) |
| + | 22 (84.6) | 3 (10) | 20 (76.9) | 11 (37.9) | 1 (3.8) | 12 (40) | 1 (3.8) | 1 (3.3) |
| ++ | 3 (11.5) | 17 (56.7) | 0 (0) | 15 (51.7) | 2 (7.7) | 7 (23.3) | 2 (7.7) | 3 (10) |
| +++ | 0 (0) | 9 (30) | 0 (0) | 1 (3.4) | 0 (0) | 4 (13.3) | 0 (0) | 1 (3.3) |
| P Value | 0.000 | 0.000 | 0.000 | 1.000 | ||||
Abbreviations: GTN, Gestational Trophoblastic Neoplasia; SMP, Simple Molar Pregnancy.
aFor p53 marker, we applied 0 for no stained cells, + for staining of less than 10% of cells, ++ for 10 to 50% of cells, +++ for staining in more than 50% of cells. To score c-erbB-2 staining intensity we used negative as of no or less than 10% of cells’ membranes stained, + for faint membranous staining in more than 10% of cells, ++ for weak to moderate complete membranous staining in more than 10% of cells and evaluate +++ as strong, for complete membranous staining in more than 30% of cells, results of chi-square test.
bP value < 0.05 considered significant.
cValues are expressed as No. (%).
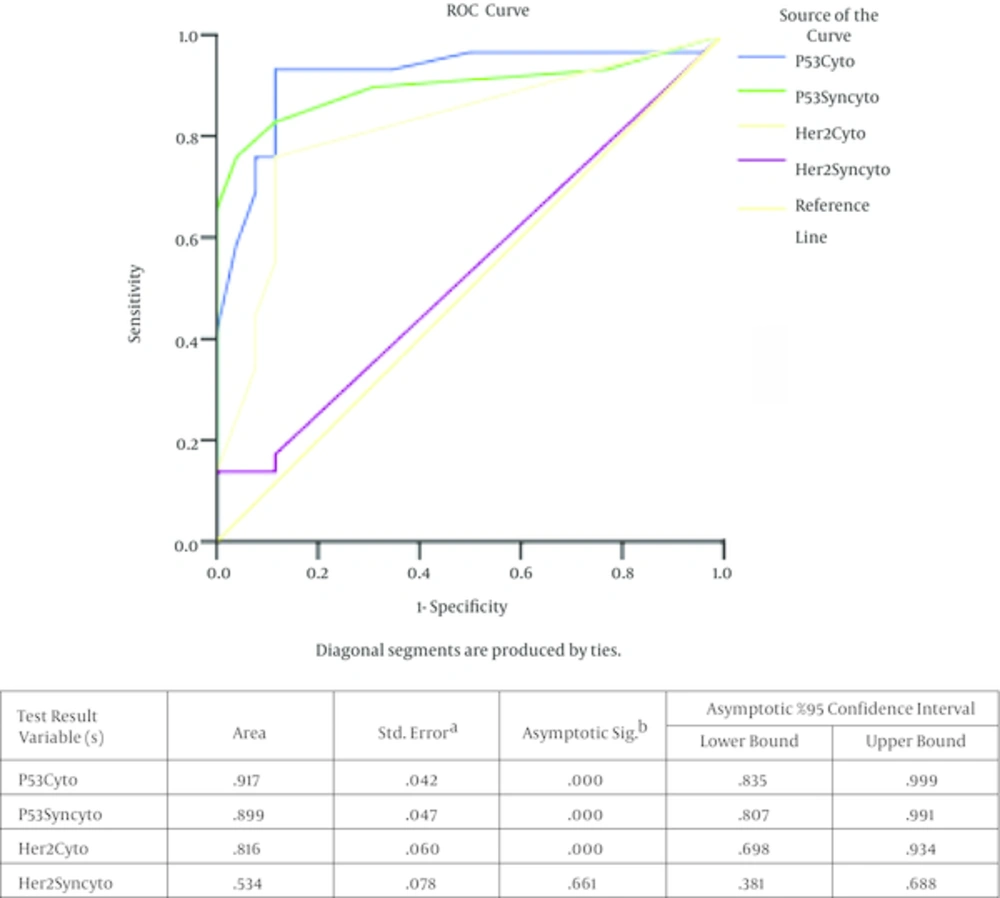
The receiver operating characteristic (ROC) curve analysis displayed the 5.5% as a cutoff percentage for cytotrophoblast with p53 nuclear immunostaining (93.3% sensitivity, 88% specificity). For syncytotrophoblast with sensitivity and specificity of 90% and 88% respectively, the cutoff value of 2.5% was determined. We found the cut off value of 12% for the percentage of cytotrophoblast with c-erbB-2 membranous staining (sensitivity of 90% and a specificity of 92%) which might increase the risk of progression of a molar pregnancy to GTN (Figure 2) .The positive predictive values were as 90%, 88.8% 88.4% for calculated cut off of p53-positive cytotrophoblast, p53 positive syncytiotrophoblast and c-erbB-2 positive cytotrophoblast respectively (Table 4).
For p53 and c-erbB-2 markers among cytotrophoblasts and syncytiotrophoblasts in molar tissue to estimate their risk to GTN transformation.The test result variable(s): p53Cyto, p53Syncyto, Her2cyto, Her2Syncyto has at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased. a, Under the nonparametric assumption. b, Null hypothesis, true area = 0.5.
| P53 Cytotrophoblasts, (%) | P53 Syncytiotrophoblast, (%) | C-erbB-2 Cytotrophoblasts, (%) | |
|---|---|---|---|
| Cut off for percentage of cells with positive immunostaining | 5.5 | 2.5 | 12.5 |
| Positive predictive value | 90 | 88.8 | 88.4 |
| Negative predictive value | 92 | 82.1 | 76.6 |
5. Discussion
In patients with simple molar pregnancy, gynecologists are always concerned about their progression toward the gestational trophoblastic neoplasia. To date the only way to evaluate patients following the evacuation and curettage, is serial measurement of serum βhCG (11). Regarding the invasive and metastatic behavior of malignant transformations of molar pregnancy, finding a marker with high predictive value to diagnose the malignant forms early after evacuation is of great importance. In this survey, we found that p53 and c-erbB-2 genes had higher expressions in both cytotrophoblast and syncytiotrophoblast of GTN patients in comparison with simple molar patients with significant difference.
P53 is known as a tumor suppressor gene which encodes a nuclear phosphoprotein and its mutation seems to involve in many human cancers’ pathogenesis (13, 19, 20). Several studies have been performed on the role of p53 in gestational trophoblastic neoplasia. Petignat et al. reported the over expression of mutant p53 in complete moles and malignant forms (21). Although many studies reported an increase in p53 expression in GTN and complete mole in comparison with simple molar pregnancy and partial mole (12, 14 - 16, 22-24), some found that the increased type is rather the wild type to the mutant type of p53 (12, 23, 25, 26). Yang et al. also reported an increased expression of p53 in GTN and complete mole although he did not find a significant predictive value for such increase to diagnose the malignant forms in early stages (16). Whether the mutant type or wild type are over expressed, in this survey we found that p53 expression increased significantly in cytotrophoblast and syncytiotrophoblast of GTN and complete moles. We found the positive predictive values of 90% and 88.8% when 5.5% and 2.5% of cytotrophoblast and syncytiotrophoblast with positive nuclear immunoactivity were used as the cut off respectively. Using the same method of immunostaining, Chen Y et al. results support our data and find it useful to evaluate p53 expression as adjuncts to conventional methods of diagnosis (24).
Epidermal growth factor receptors (EGFRs) are a big family of transmembrane signaling proteins which are involved in many human neoplasms pathogenesis (27-29). C-erbB-2 is a member of (EGFRs) family and is involved in pathogenesis of different malignancies including melanomas, breast cancer, and colorectal cancer (30, 31) as well as complete mole and choriocarcinoma (16, 27, 32, 33). Yang et al. reported a prognostic value of 84% for percentage of cytorophoblasts with positive cytoplasmic immunostaining to predict the malignant progression of simple molar pregnancy (16). However, there are conflicting reports: Cameron et al. reported the expression of c-erbB-2 in only one case out of 20 patients with persistent gestational trophoblastic disease (34); Dehaghani AS et al. found that the mean serum c-erbB-2 does not differ significantly between GTD patients and normal pregnant controls (35). In contrast to these reports, we found an over expression of c-erbB-2 in cytotrophoblast of patients with GTN and complete mole with significant difference to simple molar pregnancy and partial mole. We determined a cut off value of 12.5% for the percentage of cytotrophoblast with c-erbB-2 membranous staining (sensitivity of 90% and a specificity of 92%) which might increase the risk of progression of a molar pregnancy to GTN. Many researchers have supported our data: Yang et al. has found c-erbB-2 a strong predictor for malignant behavior of molar pregnancies; Yazaki et al. proposed to use c-erbB-2 expression as well as βhCG in therapeutic protocols (16, 27, 32, 33, 36).
Our data is noteworthy because of the more accurate method of immunohistochemistry we have used; unlike the most studies on this issue using avidin-biotin methods (16, 23, 32, 33, 37, 38), our study was performed on EnVision Tm system. Avidin-biotin methods are widely used since their introduction in 1981 but because of the background staining due to tissues endogenous biotin and decreasing the expression of biotin in formalin fixation and paraffin blocking of tissues, the new method of polymer based methods have been established (39, 40). EnVision Tm system is a new polymer based technique which has higher sensitivity than routine avidin-biotin method without its limitations (41).
Although our study was performed only by one pathologist and was not a blind study, using a more accurate technique of immunostaining and counting immunostained cells separately on each cell population (cytotrophoblast and syncytiotrophoblast) might make our results more practical to be a base for future studies. Finally, further collaboration of pathologists and gynecologists would be suggested to establish comprehensive guidelines for early diagnosis of malignant progression of molar pregnancies.
Our data suggests that over expression of p53 and c-erbB-2 is associated with malignant progression of molar pregnancy. We encountered that high expression of p53 and c-erbB-2 in trophoblastic cells could predict gestational trophoblastic neoplasia in early stages. Supposed our data could be supported with more studies on this issue, it might be useful to evaluate the immuno-expression of p53 and c-erbB-2 genes on primary samples of pregnancy products of patients with molar pregnancy to estimate their risk of progression toward the malignant forms.