1. Introduction
Stevens-Johnson syndrome (SJS) has been an immune-mediated hypersensitivity reaction that involved the mucous membranes and skin in which detachment of 10% of the BSA occurs. Typical prodromal symptoms of SJS were arthralgia, malaise, fever, body aches, headache, fever, epistaxis, and tachycardia, and then complications such as a burning rash, have been beginning on the face and upper part of the torso (1). The rash could begin as macules, bullae, or confluent erythema. Lesions might later rupture, leaving denuded skin, which could become susceptible to secondary infection. The most common areas that might involve with this disease include the dorsum of the hands, palms, and soles. Signs of mucosal involvement could include erythema, edema, blistering, and ulceration. The most common pattern of mucous membrane involvement of this disease include oral, eye (dry eye, red eye, and tearing), gastrointestinal, urethral, vaginal; other areas such as the lower respiratory tract might involve during the course of the illness. Histopathology of the skin has shown changes in the epidermal-dermal junction, ranging from vacuolar alteration to sub epidermal blisters. The death of keratinocytes has caused separation of the epidermis from the dermis. The four etiologic categories have thought to be causes of SJS: Idiopathic (as many as half of cases), drug-induced, malignancy-related, collagen- and vascular-related. In differential diagnosis, SJS which has involved less than 10% toxic epidermal necrolysis (TEN) (2), involved detachment of the epidermis and erosions of more than 30% of the body surface. Overlap of SJS and TEN (SJS-TEN) has characterized by shaped, erythematous, or purpuric macules with blistering that becomes confluent and resulted in detachment of the epidermis on 10% - 29% of the body surface area. Most patients with SJS have treated symptomatically. The principle of treatment had three stages. In the primary stages, all suspected drugs have stopped (3). In the second stage, patients should be treated with special attention to airway and hemodynamic stability, given fluids and antibiotics, electrolyte management (4, 5), and wound care (6-8). In the third stage, areas of denuded skin must be covered with compresses of saline.
2. Case Presentation
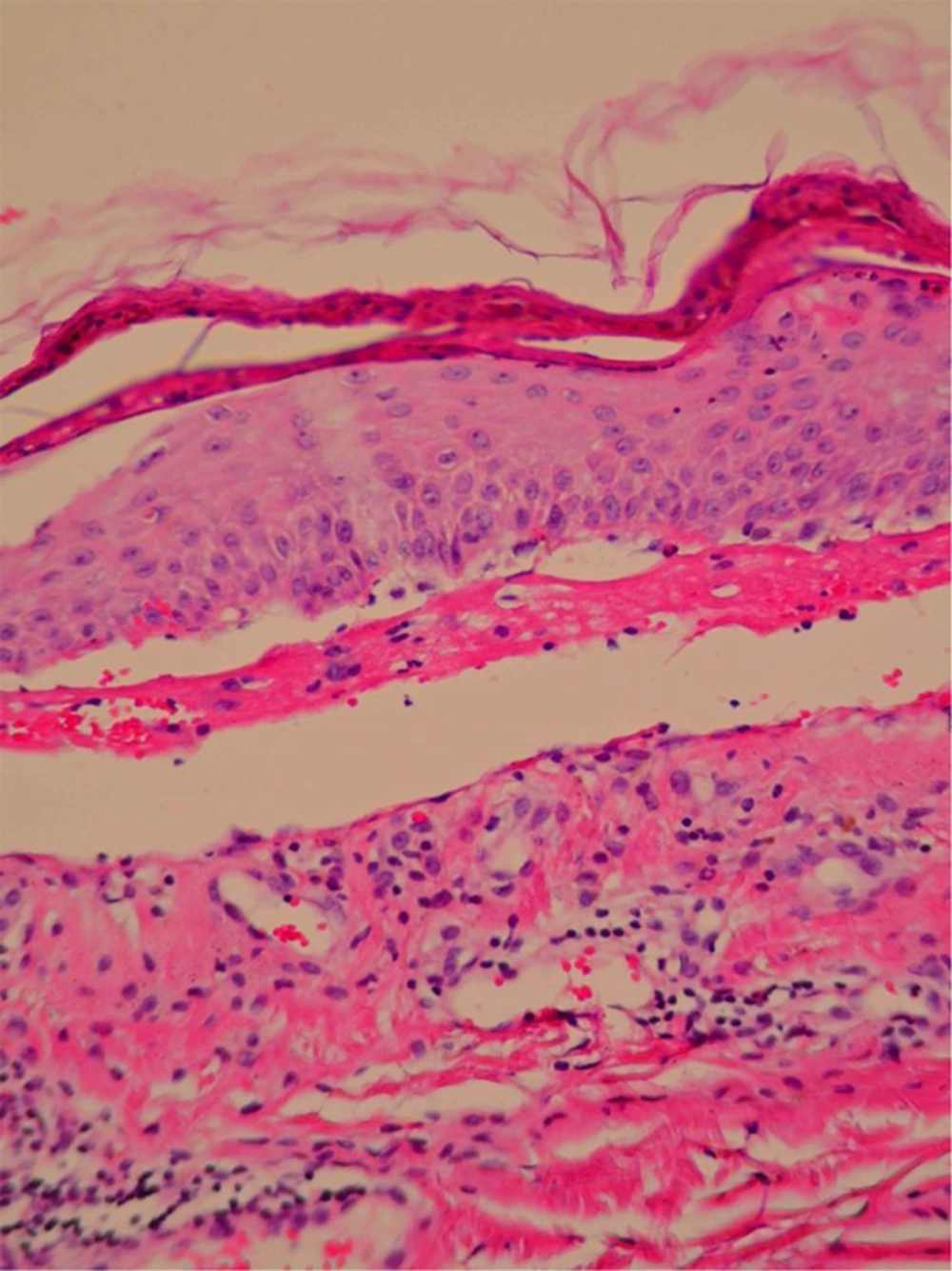
We have treated a 54-year-old woman with biliary tract cancer. The patients has received chemotherapy consisting of cycles of continuous infusion of 5-FU for 21-hours (600 mg/m2) days 1, 2, 3, and 4. Gemcitabine has given at dose of 1,250 mg/m2 days 1 and 8 and cisplatin has given at dose of 60 mg/m2 day 1 with G-csf every support. Each cycle has repeated every 21 days for 6 cycles (9). After her second exposure to combination chemotherapy, she has presented with arthralgia, malaise, fever, body aches, headache, and sore throat, and two days later, an epidermal detachment has appeared in 10% of the total body surface area, presenting as blistered skin on the legs and posterior chest. Involvement of the oral mucosa has occurred too with rapidly evolving vesiculobullous eruption in genitalia. Skin biopsy has done and the pathology has shown extensive epidermal necrosis, focal subepidermal necrotic blisters, and separation of the epidermis from the dermis (Figure 1). She has admitted to the oncology ward and sepsis workup has done, followed by infusion of empirical intravenous broad spectrum antibiotics. The other supportive care has included wound care, fluid and electrolyte management, nutritional support, ocular care, temperature management, pain control, and monitoring for/treatment of super infections. All dermal and mucosal lesions have healed without scarring after 23 days, and then have initiated other regimen chemotherapy.
3. Discussion
The mechanism underlying SJS has remained unknown in patients with combination chemotherapy. The agents of chemotherapy, such as antimetabolites or alkylating agents, including cytarabine, methotrexate, and 5-fluorouracil interfere with RNA or DNA synthesis. But this problem has not happened with all these drugs; it has occurred in a very small percentage of patients who use this drug, and therefore other factors such as genetic predisposition in these patients should consider (10, 11). .Drug-induced SJS typically begins 1 - 3 weeks after therapy initiation. The patients in general have received several drugs possibly involved in SJS pathogenesis. We have believed that our case of SJS has related to one of three drugs, gemcitabine, cisplatin, or 5-FU or a combination of the three drugs because no convincing alternative explanation has proposed. Stevens-Johnson syndrome have been reported in patients treated with any of the drugs gemcitabine, cisplatin, 5-fluorouracil individually but very rarely (less than 0.01% to 0.1%).Several reports have described SJS induced by other anticancer agents (1, 12). This case has served as an alert for the need to observe patients closely for potentially dangerous cutaneous reactions to these three drugs. Other hypotheses, therefore, must consider. As the first suspicious sign has observed, drug therapy should discontinue immediately and aggressive medical management should initiate.
3.1. Conclusion
Although SJS has been a rare toxicity, it should always consider with concurrent administration of 5-FU, gemcitabine, and cisplatin. If a triple therapy has employed, they must administer with caution and all subsequently developing cutaneous reactions must promptly evaluate with a high index of suspicion for SJS.