1. Introduction
Choriocarcinoma has been known as an aggressive neoplastic disease and usually has an coexisting antecedent pregnancy. This type of neoplasm can be diagnosed based on histology of previous pregnancy (molar or non-molar) (1). Choriocarcinoma may not be initially diagnosed in patients with an established menopause, especially when there is no evidence of metastatic neoplasm; therefore, the occurrence of choriocarcinoma in postmenopausal women is very rare (2, 3). Rangwala et al. in 2011, reported that It is a rare disorder with an incidence of 1 in 400 in Asia and Latin America (4). Also, Samal et al. in 2014 reported that gestational choriocarcinoma has an incidence of one in 20,000 to one in 25,000 in western countries (5). In addition, the long latent period between development of a choriocarcinoma and the last pregnancy is reported in some studies (6). Choriocarcinoma has been reported in a 73-year-old postmenopausal woman 38 years after pregnancy (7). O’Neill et al. reported uterine choriocarcinoma developing after a long latent period (22 years) in a postmenopausal woman after last pregnancy (8). 75 to 95% of choriocarcinoma cases can be cured despite distant metastasis; therefore, acurate diagnosis is very important. Also, these patients should be managed by multidisiplinary and experienced team (9).
This article reports three cases of postmenopausal choriocarcinoma with different clinical signs and symptoms.
2. Case Reports
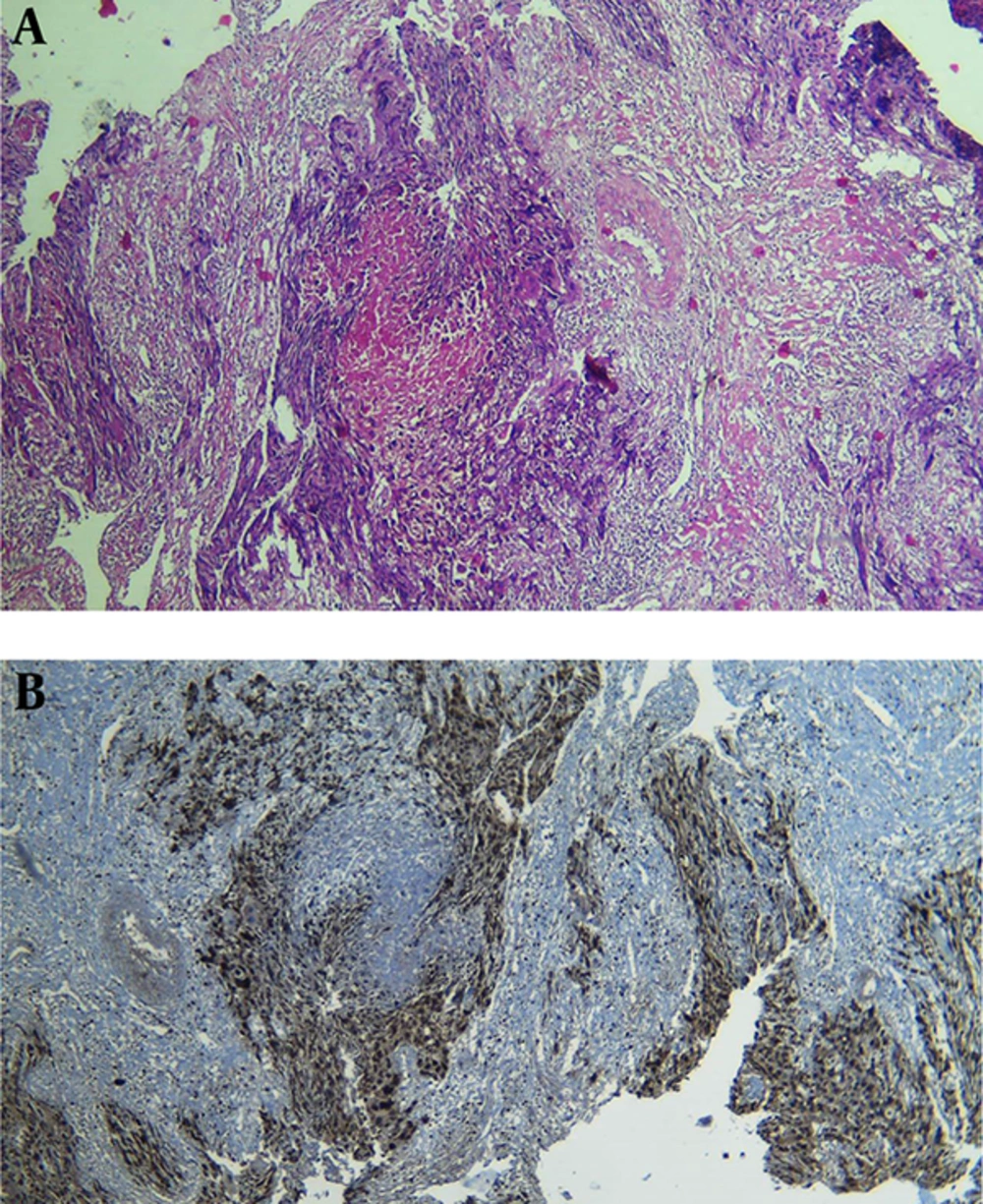
Case 1: A 60-year-old multiparous woman was referred to the department of gynecology-oncology at Ghaem Hospital, Mashhad University of Medical Sciences, Iran in 2015. Her symptoms were non-bloody vaginal discharge and a mass with size of 2 × 3 cm in suburethral of vaginal wall. Her last pregnancy was 13 years before which ended the pregnancy term. She had no history of molar pregnancy or abortion. Clinical examination revealed large uterus as 10 weeks of gestation. Biopsy was performed due to suspicion of vaginal mass to melanoma. Histological features and immunohistochemistry were consistent with choriocarcinoma (Figure 1). Serum β-hCG level was 565,000 mIU/mL. Chest X-ray revealed several lesions. Finally, multiagent chemotherapy with EMA/CO regimen was initiated with diagnosis of FIGO stage III according to WHO classification (on the first day: Etoposide 100 mg/m2, Methotrexate 100 mg/m2 IV buluse, and then 200 mg/m2 over 12 hours, and Actinomycin 0.5 mg; on the secound day: Etoposide 100 mg/m2, Actinomycin 0.5 mg and Folinic acide 15 mg every 12 hours for 48 hours; at 8th day: cyclophosphamide 600 mg/m2 and vincristine 1 mg/m2). β-hCG level returned to normal following 5 cycles of chemotherapy regimen. This regimen continued until two subsequent normal level of β-hCG. Her response to chemotherapy was confirmed by decreased β-hCG level.
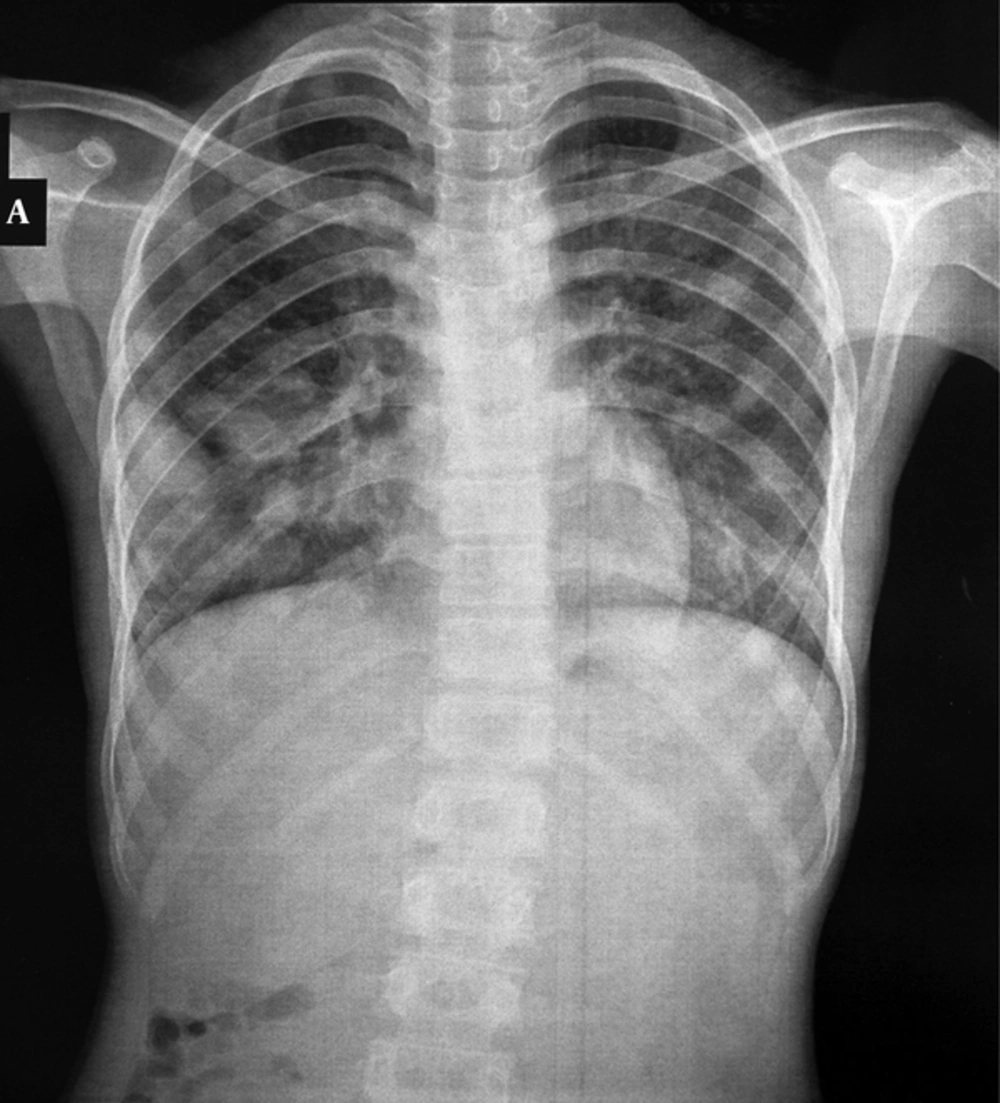
Case 2: A 54-year-old postmenopausal woman was referred to the same hospital with a history of vaginal bleeding three years after menopause and histological report of choriocarcinoma in endometrial curetage. β-HCG level was 1,250,000 mIU/mL. CT–Scan detected multiple pulmonary and liver metastasis (Figure 2). Combination chemotherapy with EMA/CO regimen was initiated based on FIGO stage IV. She achieved complete remission after seven cycles of chemotherapy regimen.
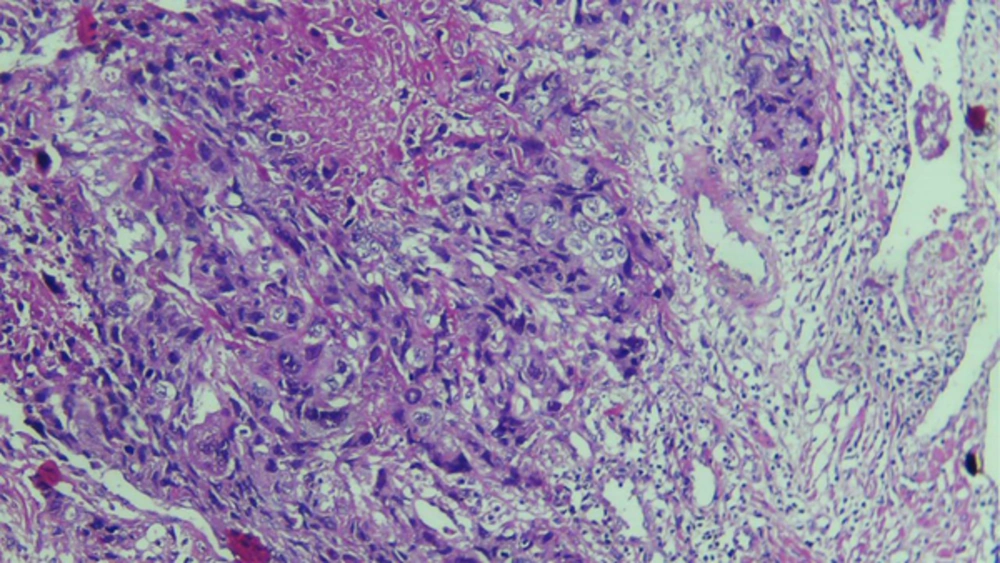
Case 3: A 48-year-old postmenopausal woman was referred to the same hospital with history of amenorrhea from five years before. Her chief complaint was vaginal bleeding. The histological features of endometrial biopsy were consistent with choriocarcinoma. Serum β-hCG level was 15,000 mIU/mL (Figure 3). Single agent chemotherapy with methotrexate 50 mg/m2 /week was initiated with diagnosis of choriocarcinoma and FIGO stage I of gestational trophoblastic neoplasm. β-HCG level was normal after three courses of chemotherapy regimen.
The three cases have been regularly followed-up and acheived serological remission until now.
An informed consent was obtained from each participant for reporting their data in this study.
3. Discussion
Choriocarcinoma is considered as the most curable gynecologic cancer even in the presence of metastatic disease. The possibility of choriocarcinoma should be considered in postmenopausal women and if occurred, management of these patients should be initiated as soon as possible. This means that early diagnosis of the disease and referring to an experienced center are associated with decreased morbidity and mortality. Gestational trophoblastic neoplasia is usually followed by secondary antecedent pregnancy, especially choriocarcinoma is very rare after menopause (10). The incidence of choriocarcinoma depends on the geographical area, its incidence is 1 per 1000 live births in developed countries (11, 12). Also, it is estimated that in our center 0.3% of GTN patients had postmenopausal choriocarcinoma. In literature review, there are very few case reports of gestational diseases in postmenopausal women. Garcia et al. in a study reported that 109 cases of GTD in women older than 50 years were evaluated in the larger historical series and found malignant disease in 28.4% and benign moles in 47.7% (13). The occurrence of pregnancy may be possible but without clinical symptoms. Desai et al. in their review of the literature reported a case of choriocarcinoma in a 73-year-old woman developing 38 years after the last pregnancy and 23 years after menopause (14). Moreover, there are reports of a very long latent period between last pregnancy and occurrence of choriocarcinoma with unknown mechanism (9, 14) (Table 1). In the present study, the maximum duration between last pregnancy and occurrence of choriocarcinoma was five years. Evsen et al. reported a rare case of postmenopausal bleeding with a history of a molar pregnancy three years after menopause at 52 years, and subsequently choricarcinoma was developed at 58 (15). Clinical signs or symptoms of choriocarcinoma in postmenopausal period are not specific. Postmenopausal vaginal bleeding or secondary metastatic manifestation may be the first chief complaint of these patients. In this study, the most complications of the patients were postmenopausal vaginal bleeding and discharge. Vaginal bleeding was the most common symptom in three cases, so postmenopausal vaginal bleeding is not always the indication of ovarian, cervix, and endometrial cancers. However, pelvic ultrasound and β-hCG titrage mostly help to the diagnosis. Choriocarcinoma is extremely chemo-sensitive, approximately an estimated rate of nearly 90% with available chemotherapy regimens (15). Also, in the study of Bazinet et al. they could not rescue the patient due to poor conditions (16), but in our study, we found response to chemotherapy based on decrease in serum tumor markers that suggested effective management of choriocarcinoma by EMA/Co regimen in advance stage and Methotrexate in early stage of disease like some other studies (17). In our patients, maximum serum β-hCG was 565,000 mIU/mL and despite stage III of disesase and they were cured with chemotherapy, but Desai et al. found chemotherapy response rate of 2,704,040 and stage IV of GTN (14). The maximum courses of chemotherapy in our study was seven courses that is simmilar to the study of Desai et al. (14).
| Age, y | Latent Long of Menopause, y | β-hCG Level, mIU/mL | Stage of Disease | Chemotherapy Regimen | Courses of Chemotherapy | ||
|---|---|---|---|---|---|---|---|
| Our cases | Case 1 | 60 | 5 | 565,000 | III | EMA/CO | 5 |
| Case 2 | 54 | 3 | 1,250,000 | IV | EMA/CO | 7 | |
| Case 3 | 48 | 5 | 15,000 | I | Methotrexate | 3 | |
| Other cases | Kaabia | 63 | 14 | 33,652 | I | EPA | 5 |
| Desai | 73 | 23 | 2,704,040 | IV | EMA/CO | 7 | |
| Bazinet | 56 | 2.05 | - | IV | Dead | - |
In this study, we did not have any restrictions in the follow-up of the patients.
3.1. Conclusions
Choriocarcinoma can occur in women at all reproductive ages and rarely occurs in postmenopausal age. A high level of possibility of postmenopausal choriocarcinoma may help to the diagnosis of this rare event. The possibility of diagnosis of this neoplasia should be considered because extreme response to chemotherapy even with metastatic disease.