1. Background
Melanin, a pigment produced by melanocytes, is responsible for coloring mammalian hair and skin. Melanin formation (melanogenesis) is a very complex process and is regulated by enzymatic and non-enzymatic pathways. It begins with tyrosinase mediated oxidation of l-tyrosine to l-DOPA. This is followed by tightly controlled series of oxidoreduction reactions that involve production of some toxic and mutagenic compounds such as quinones, semiquinones and reactive oxygen species (ROS). Afterwards, tyrosinase over activity increases some toxic and mutagenic intermediates of melanogenesis as well as hyperpigmentation. Moreover, it can affect the melanoma cells’ behavior through autophagy effect on tumor cells (1-3). The melanin can protect malignant melanocytes as well as normal melanocytes from numerous physical and chemical damages. On the other hand, over-exposure to UV radiation increases the tyrosinase activity via generation of reactive oxygen species (ROS). ROS can cause extensive cellular damage and decrease enzymatic antioxidant defense. Based on these observations, melanogenesis is involved in several human skin disorders, including pigmentation disorders and melanoma (3-5). Therefore, suppressing melanogenesis through inhibition tyrosinase activity together with increasing antioxidant status can be an effective strategy to achieve both anti-melanogenic and anti-melanoma properties.
Numerous studies have shown that some plant polyphenols influence the melanin biosynthesis and growth of various melanoma cells through their anti-tyrosinase activity as well as antioxidant effect (4, 5). The genus Phlomis (Lamiaceae) is rich with polyphenols (flavonoids and phenylethanoids/phenylpropanoids), iridoids and essential oils. A number of Phlomis species revealed various pharmacological activities such as antioxidant, antimicrobial, immunosuppressive, anti-mutagenic, anti-cancer, anti-diabetic and anti-inflammatory effects (6, 7). Previous studies have described the cytotoxicity and anti-tumor activity of several Phlomis species such as P. lanceolata and P. russeliana (8, 9). Recently, Salimi et al. confirmed that the cytotoxicity and anti-melanogenesis activity of P. kurdica extract on SKMEL-3 cell line (10).
Phlomis caucasica, an endemic species from Iran, has been used in the treatment of throat infections in traditional Iranian medicine (TIM). According to a previous study (11), some flavonoids and phenylethanoid/phenylpropanoid glycosides have been identified from methanol fraction of the aerial parts of P. caucasica. Of these compounds, only forsythoside B and acteoside have shown excellent antioxidant activity via DPPH free radicals scavenging assay. On the other hand, two isolated flavonoids, naringenin and kaempferol 3-O-glucoside, have shown very low antioxidant and anti-tyrosinase activity (12). Moreover, some iridoid glycosides were found in this species that have been shown to have opposing effects on melanogenesis, for example geniposide enhanced melanogenesis, while loganin has indicated anti-melanogenic activity (13, 14). Therefore, due to the presence of various compounds in P. caucasica, with opposing and complementary effects on melanin production, it can be involved in melanogenesis pathways.
2. Objectives
The present study was designed to investigate the anti-melanogenic and cytotoxic activities of MeOH extract from P. caucasica (MPc). We evaluated the level of antioxidant and anti-tyrosinase capacity of MPc by DPPH radical scavenging and mushroom tyrosinase activity assays, respectively. In addition, the effects of MPc on melanin content, cellular tyrosinase activity and cytotoxicity were determined in the human melanoma SKMEL-3 cells.
3. Methods
3.1. Chemicals
Folin–Ciocalteu reagent was obtained from Fluka (Buchs, Switzerland). Tyrosinase from mushroom, butylated hydroxytoluene (BHT), 1, 1-diphenyl-2-picrylhydrazyl (DPPH), kojic acid, quercetin, dimethyl sulfoxide (DMSO), sodium carbonate, potassium dihydrogen phosphate, di-potassium hydrogen phosphate and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma, the US. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin, streptomycin and trypsin-EDTA were obtained from GibcoBRL, the US. Human melanoma cells (SKMEL-3) were purchased from Pasteur Institute in Iran. All other chemicals and solvents used in experiments were analytical grade and obtained from Merck (Germany).
3.2. Plant Material and Preparation of the Extract
The aerial parts of Phlomis caucasica Rech.f. were collected from the Ahar in East Azerbaijan, Iran and were identified by Mr. Yousef Ajani. A voucher specimen (No. 1648 ACECR) was deposited at Herbarium of Medicinal Plant Institute, Academic Center for Education, Culture and Research, Karaj, Iran. The dried powder of P. caucasica (100 grams) was extracted with 80% MeOH using percolation apparatus. After filtration, the solvent evaporated in vacuum and concentrated with freeze-dryer. The dried extract (yield 4.63%) was stored at 4°C for experimental studies.
3.3. Determination of Total Phenolic Content
Total phenolic content of the extract was determined according to the Folin- Ciocalteu method (15) with some modifications. Folin-Ciocalteu reagent (100 μL) was added to the sample and after 5 minutes, the mixture was treated with 0.75 μL of sodium carbonate 6% (w/v) and was left for 30 minutes. The absorbance was measured at 750 nm using an ELISA reader (Synergy HT, BIO-TEK, USA). The amount of total phenolic content of the sample was expressed as milligrams of gallic acid (GA) equivalents per gram of the dry extract.
3.4. Determination of Total Flavonoid Content
Total flavonoids content of the extract was estimated as the aluminum chloride colorimetric method by 96-well plate (16). Twenty μL of the extract was mixed with water (80 μL) followed by the addition of NaNO2 (15%, 6 μL). After shaking, 6 μL of 10% aluminum chloride (AlCl3), 80 μL of NaOH (4%) and 80 μL distilled water were added. After 15 minutes, the absorbance of mixtures was measured at 510 nm with the ELISA reader (Synergy HT, BIO-TEK, USA). Total flavonoid contents were calculated as quercetin (Q) equivalents per gram of the extract.
3.5. Determination of DPPH Radicals Scavenging Effect
The technique using 96-well microplate was applied to determine the DPPH free radicals scavenging activity of the extract (17). Methanolic DPPH solution (150 µM) was prepared and the solution was kept in the dark at 4°C. Different concentrations (160 µL) of the extract (0.001 - 10 mg/mL) were mixed with 40 µL of DPPH solution. The plate was shaken and then incubated at 25°C for 30 minutes in a dark place. The UV absorbance was recorded at 517 nm using an ELISA reader. Inhibition percentage was calculated using the following formula: DPPH radical scavenging activity (%) = [(A - B)/A] × 100; where A = absorbance at 517 nm without test sample and B = absorbance at 517 nm with test sample. BHT was used as a positive control. A dose response curve was plotted to determine the SC50 value. SC50 is defined as the concentration of sample adequate to obtain 50% of a maximum scavenging capacity.
3.6. Determination of Mushroom Tyrosinase Inhibitory Activity
Tyrosinase inhibitory activity was carried out according to the procedure as explained by Sarkhail et al. (14, 16). The extract was dissolved in dimethyl sulfoxide (DMSO) to make the test concentrations (0.001 - 10 mg/mL). A mixture of 80 μL phosphate buffer (50 mM), 20 μL of mushroom tyrosinase solution (125 units/mL in 50 mM phosphate buffer, pH 6.8) and 40 μL of sample solution was pre-incubated at room temperature for 10 minutes. Finally, a reaction was performed by adding 40 μL L-tyrosine (2 mM) for 15 minutes at room temperature. The amount of dopachrome in the reaction was quantified at 475 nm using an ELISA reader. The percentage of inhibition of tyrosinase was calculated as follows: tyrosinase inhibition (%) = [(A - B)/A] × 100; where A = absorbance at 475 nm without test sample and B = absorbance at 475 nm with test sample. Kojic acid was used as a positive control. The concentration at which half the original tyrosinase activity is inhibited (IC50) was determined for each sample.
3.7. Cell Culture and Treatment
Human malignant melanoma cells (SKMEL-3) were obtained from cell bank of Pasteur Institute, Iran (NCBI). Cells were cultured in DMEM containing 10% fetal bovine serum, 100 U/mL penicillin G, 100 mg/mL streptomycin and maintained at 37°C, 95% humidity and 5% carbon dioxide. For anti-proliferative studies, SKMEL-3 cells were plated at a density of 5 × 103 cells/well in 96-well plates. After 24 h of incubation, various doses of the extract (0.001 to 0.5 mg/mL) were added.
3.8. Cytotoxicity Assay
Cytotoxicity was measured using the 3-(4, 5-dimethyl-thiazol-2-yl)-2, 5 diphenyltetrazolium bromide (MTT) reduction method (18). After drug treatments, cells were incubated with MTT solution (5 mg/mL) for 4 hours at 37°C. The formazan crystals were dissolved in DMSO and the color violet was measured at 545 nm using microplate reader. Solvent control (DMSO) was included to check if the DMSO had any effect on the concentration used.
3.9. Melanin Content Assay
Melanin content was measured as described previously by Chan et al. (4) with slight modifications. The human melanoma cells were seeded with 3 × 105 cells/ well in 3 mL of medium in 6-well culture plates and were incubated overnight to allow cells to adhere. The cells were exposed to various concentrations of the extracts for 72 hours. At the end of the treatment, the cells were washed with PBS and lysed with 800 μL of 1 N NaOH (Merck, Germany) containing 10% DMSO for 1 hours at 75°C. The melanin content was determined using a microplate reader at 405 nm absorbance.
3.10. Cellular Tyrosinase Activity Assay
For cellular tyrosinase activity assays (4), SKMEL-3 cell (2 × 105 cells/well in 3 mL of media in 6 well plates) were incubated overnight in a humidified CO2 incubator at 37°C and 5% CO2 to allow cells to adhere. The cells were treated with increasing concentration of test extract for 72 hours. Finally, the cells were washed with PBS (pH 6.8) and lysed with mammalian protein extraction (M-PER) reagent. The lysates were then clarified by centrifugation at 13,000 rpm for 15 minutes at 4°C. The protein concentration was evaluated by the Bradford using bovine serum albumin as the standard. The reaction mixture containing 40 μg protein (adjusted to 100 μL with 0.1 M PBS, pH 6.8) and 100 μL of 5 mM l-Dopa was added to each well of a 96-well plate. After incubation at 37°C for 1 hour, the absorbance was determined at 475 nm with a microplate reader. Tyrosinase activity in the protein was calculated by the following formula: tyrosinase activity (%) = [OD475nm of sample/OD475nm of control] × 100.
3.11. Statistical Analyses
The results were reported as mean ± SEM and all experiments were performed in triplicate. Data subjected to independent t tests and the group means were compared using the ANOVA followed by Tukey post hoc test. P < 0.05 was considered as statistically significant. All analysis performed using Graph Pad Prism 6.
4. Results
4.1. Total Phenolic and Flavonoid Contents of MPc
The content of total phenols of P. caucasica methanol extract was measured by Folin- Ciocalteu reagent in terms of gallic acid equivalent (standard curve equation: y = 0.287x + 0.375, R2 = 0.999). This value was 75.84 ± 0.33 mg GA/g of dry extract. The flavonoid content of the methanol extract was measured in terms of quercetin equivalent (the standard curve equation: y = 0.124x + 0.129, R2 = 0.998) and was 28.11 ± 0.86 mg Q/g extract.
4.2. DPPH Radical Scavenging and Mushroom Tyrosinase Activity of MPc
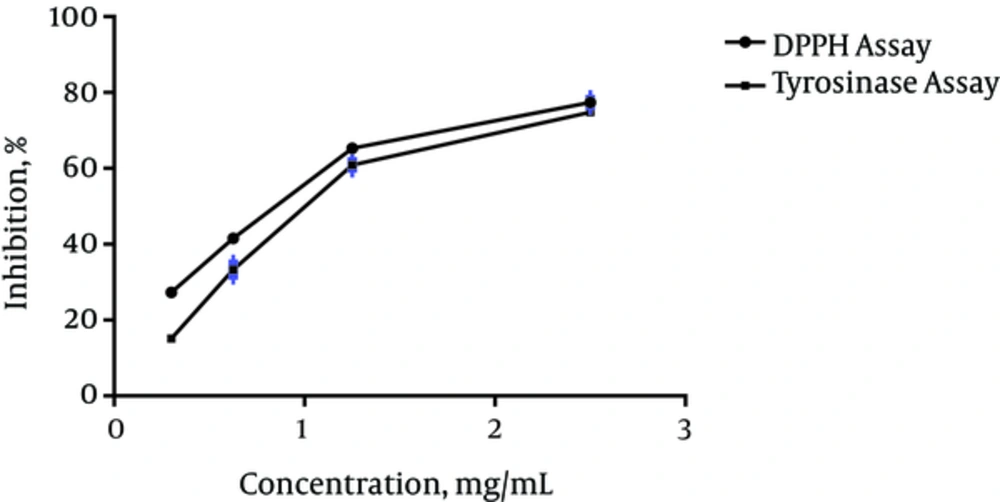
P. caucasica methanol extract exhibited scavenging activity against the DPPH radicals in a concentration-dependent manner with SC50 value of 1.037 mg/mL (Figure 1). BHT was used as a positive control in this test and its SC50 value (0.71 mg/mL) was lower than the extract. As shown in Figure 1, the inhibitory effect of P. caucasica MeOH extract on mushroom tyrosinase activity was increased with increasing sample concentrations. The IC50 of the extract was 1.316 mg/mL while the IC50 value of kojic acid (0.05 mg/mL) was far lower than that of the extract.
4.3. Cytotoxicity, Melanin Content and Tyrosinase Activity of MPc on SKMEL-3 Cells
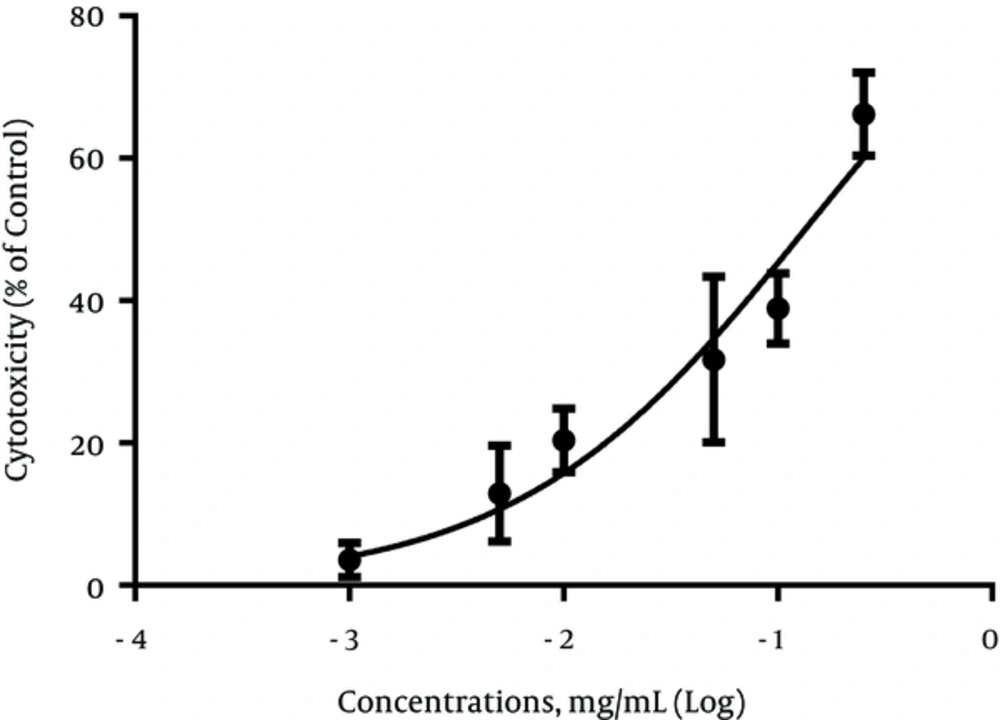
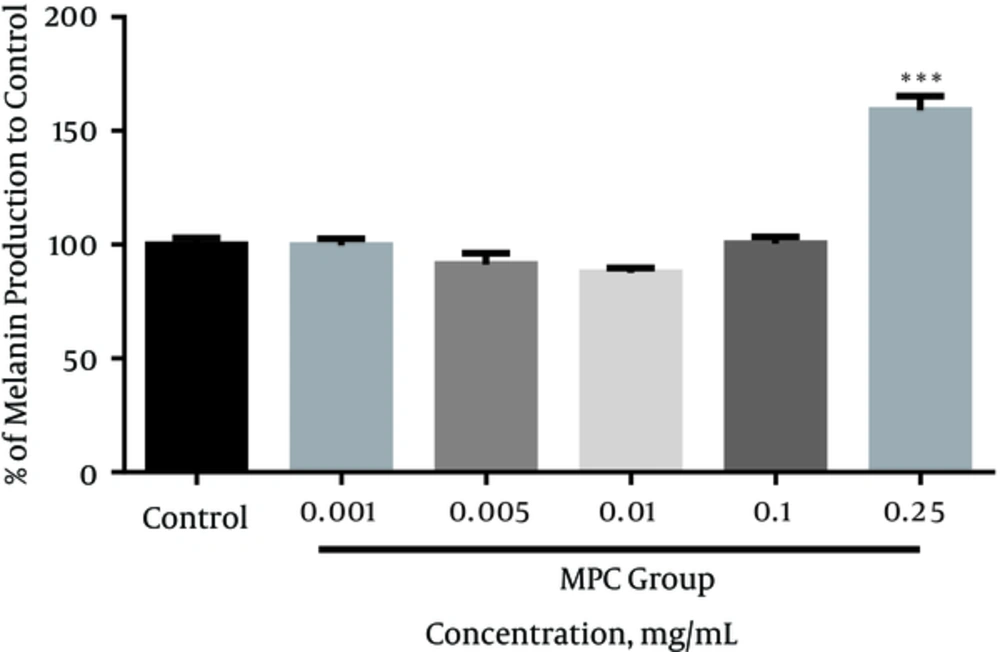
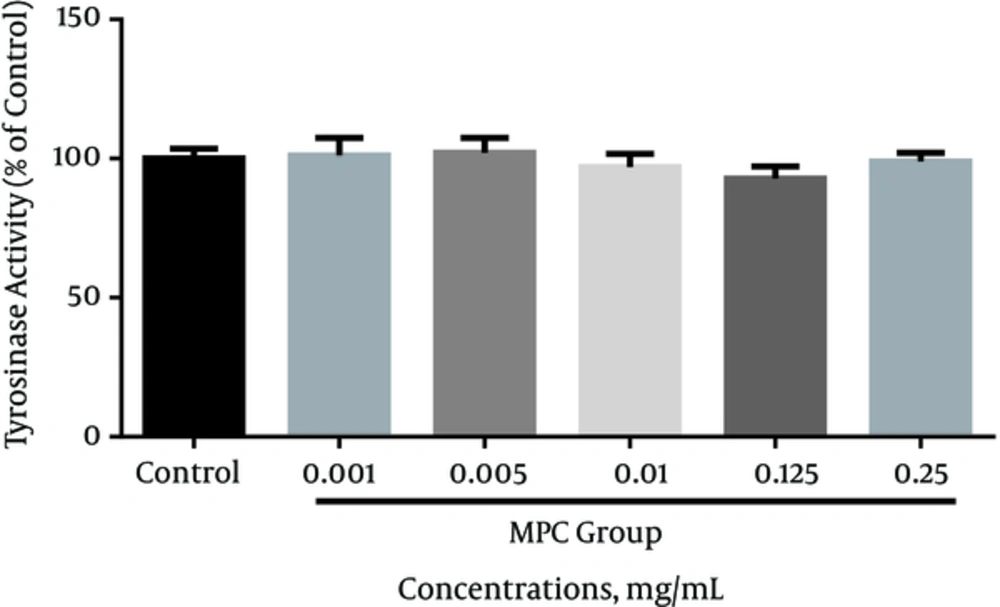
In this study, we used human melanoma cell (SKMEL-3) as the melanotic cell model in assaying the inhibitory effect of MPc on melanogenesis and cell proliferation. The cell viability in the presence of MPc was estimated by MTT assay. The data obtained indicated that the cytotoxicity significantly increased in a concentration-dependent manner compared to the control (P < 0.05). The IC50 was established as 0.134 mg/mL (95% confidence limit, 0.09791 ± 0.1834) against SKMEL-3 cells, 72 hours after treatment (Figure 2). As shown in Figure 3, the extract had no significant reduction effect on melanin content of SKMEL-3 cells in the range of 0.001 to 0.1 mg/mL when compared to control (P < 0.05). Though the melanin content significantly increased near 150% when the cells were cultured with 0.25 mg/mL of MPc. As shown in Figure 4, cellular tyrosinase activity was neither significantly decreased nor increased in cultured SKMEL-3 cells in the range of 0.001 - 0.25 mg/mL of MPc.
5. Discussions
Under normal physiological conditions, melanosomal melanin acts as a natural UV photoprotective filter for melanocytes and keratinocytes. However, after long exposure to UV radiation, melanin has shown to involve in reactive oxygen species (ROS) generation and increases the risk of developing ageing, melasma and melanoma skin cancers (5, 19).
In the present study, we aimed to evaluate the effect(s) of P. caucasica MeOH extract on the cytotoxicity and melanogenesis in SKMEL-3 cells. A number of plant polyphenols influenced the melanin biosynthesis and growth of various melanoma cells through their anti-tyrosinase activity as well as antioxidant effects (4, 5). Generally, B16-F10 murine melanoma cell line is an available model for studying the effects of extracts/compounds on melanin production. Although this cell line is similar in many melanogenic pathways with normal human melanocyte, it is different from human melanoma cell lines in several pathways (20). Accordingly, we preferred to use a human melanoma cell line for investigation of anti-melanoma and anti melanogensis activities of MPc. Many published studies confirmed that melanin can increase the resistant of melanotic melanomas to chemo-, radio- and phototherapy. Therefore, anti-tyrosinase and antioxidant compounds have been able to prevent cellular mutations and tumor cells growth and increase therapeutic effectiveness of anti-melanoma drug through melanogenesis inhibition (3, 4).
P. caucasica is rich with different glycoside compounds including flavonoids, phenylethanoids/phenylpropanoids, and iridoids. The earlier study showed that the (11) polyphenolic compounds from MeOH fraction of P. caucasica had a wide range of free radical scavenging activities in DPPH assay. For example, two phenylpropanoid glycosides, acteoside and forsythoside B, exhibited significant antioxidant activities with SC50 values between 4 to 5 μg/mL that were comparable to the Trolox® (SC50 = 2.6 μg/mL), while some isolated flavonoids from this fraction such as naringenin, kaempferol 3-O-glucoside, chrysoeriol 7-O-rutinoside, and chrysoeriol 7-O-glucoside have shown very weak antioxidant properties (518.6, 160.5, 98.6 and 94.7 μg/mL, respectively). Naringenin, as a well-known flavanone of Citrus fruits, enhanced tyrosinase activity and melanin synthesis by increasing the expression of melanogenic enzymes (21). Recently, the mechanisms underlying the activities of naringenin on melanogenesis have been investigated by Huang et al. (22). They explained that naringenin had no cytotoxic effects on B16-F10 melanoma cells at concentrations ranging from 3 to 50 μM. This compound can act as a potential tyrosinase activator and melanogenesis stimulator in B16-F10 cells by promoting the expression of tyrosinase, microphthalmia-associated transcription factor (MITF) and induce melanogenesis through the Wnt-β-catenin-signalling pathway.
In addition, iridoid glycosides, a main chemical group which are found in the genus Phlomis plants, have been shown to possess various pharmacological properties (6, 22-25). For example, geniposide, an iridoid glycoside was found in P. younghusbandii (26) enhanced melanogenesis by activating extracellular signal-regulated kinase 1/2 kinase, a downstream kinase of c-kit signaling and stimulation of stem cell factor/c-kit signaling in norepinephrine-exposed normal human epidermal melanocyte (13). On the other hand, another iridoid glycoside, named shanzhiside methyl ester, has been isolated from some species of Phlomis such as, P. younghusbandii (27) and P. rigida (28), and has shown an anti-melanogenic activity near 53.7 ± 0.41% reduction of melanin content at 100 μM in the α- MSH-stimulated B16 melanoma cells with almost no toxicity to the cells (14). Loganin and sweroside were found in some Phlomis species and indicated anti-melanogenic activity in 0.1 mM, but they were not good radical scavengers (29).
In this study, the experimental findings showed that the DPPH radical scavenging and mushroom tyrosinase activity of MPc were significantly lower than positive controls. The weak radical scavenging capacity and anti-tyrosinase activity of MPc may be due to its low polyphenolics content and/or the presence of some compounds such as naringenin with minor radical scavenging and tyrosinase stimulation effects. In addition, MPc is neither an enzymatic inhibitor nor an activator for cellular tyrosinase at concentrations ≤ 0.25 mg/mL on SKMEL-3 cells. The results have shown that MPc had no inhibitory effect on melanin formation in the range of 0.001 - 0.1 mg/mL; however, it significantly increased (P < 0.05) melanin content at 0.25 mg/mL on melanoma cells with no effect on tyrosinase activity. As the extract revealed cytotoxicity on SKMEL-3 cells at higher concentrations of 0.1 mg/mL (IC50 = 0.134 mg/mL), suggesting that, along with its melano-cytotoxic effect, MPc can increase melanogenesis in the remaining viable cells. These findings showed that MPc possibly has melanogenesis and cytotoxic activity in melanoma skin cancer.
5.1. Conclusions
The results indicated that the MeOH extract of P. caucasica contains a number of compounds with cytotoxic effect on SKMEL-3 cells. Also, some of these compounds can be involved in melanogenesis. In conclusion, to gain the therapeutic agents from MPc, further studies are needed to investigate its safety and the molecular mechanisms underlying the cytotoxic and melanogenic effects on human melanoma cells.