1. Background
Cervical cancer is the third most common malignancy in women worldwide (1) and the second cause of cancer death in women in developing countries (2). Due to health importance, several solutions have been proposed for the treatment of cervical cancer. The initial treatment of this cancer is hysterectomy, followed by chemotherapy and radio therapy. Since the human papilloma viruses (HPV), especially types 16 and 18, are known as the main causes of cervical cancer, new treatment strategies are provided based on the literature findings (3). L1 protein encoded by HPVs is the major capsid protein and stimulates the production of neutralizing antibodies in serum. This protein is an appropriate candidate for production of preventive vaccines (4). On the other hand, the viral E6 and E7 oncoproteins are constantly expressed in the tumor cells.
Some studies have shown that the HPV E7 gene alone can stimulate the immune system at intermediate levels; it may be due to a nonhuman codon bias leading to inefficient expression of viral proteins (5). It seems that in cervical cancer cells, most of produced E7 proteins are degraded rapidly by the ubiquitin-proteasome pathway; therefore, they are detectable hardly using common detection protein methods such as immunostaining, immunoprecipitation, or western blot analysis (6). On the other hand, some other studies showed that using optimized codons can improve susceptibility of target cells to E6-drived CTL (cytotoxic T lymphocyte) recognition (7). For induction of HPV-specific T lymphocytes in cervical cancer model, vaccination against determined epitopes is an attractive treatment option. Up to now, various MHC class I-restricted CTL epitopes of HPV-16 E6 and E7 have been tested in early phase clinical trials (8).
Angiogenesis inhibitors can now be considered as the fourth modality of cancer therapy (9). Endostatin is a 20-kDa C-terminal fragment derived from collagen XVIII and one of the angiogenesis inhibitors that can target more than 12% of the angiogenesis regulatory human genes. Endostatin has known as an anti-cancer drug that has neither resistance nor toxicity in humans (10). It seems that the whole endostatin protein or its derivation could be considered as a model for angiogenesis inhibitor and could be administrated with other kinds of therapeutic strategies (11, 12).
Objectives: In the current study, we examined the co-administration of a synthetic peptide derived from N-terminal loop of Endostatin and DNA vaccine containing the antigenic cellular epitopes of E6, E7 and L1 genes in cervical cancer tumor model.
2. Methods
2.1. Design of Recombinant Gene
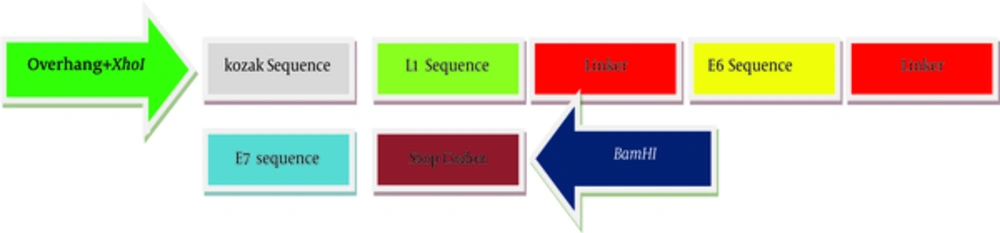
For the generation of the recombinant gene construct encoding HLA class-I (human leukocyte antigen class-I) restricted CTL epitopes, the immunogenicity of different epitops of HPV-16 E7 (49 - 57), E6 (41 - 50, 81 - 90, 98 - 107) and L1 (165 - 173) linked by a spacer of (glutamic acid, alanine, alanine, alanine, lysine) were assessed and optimized using computer-based modeling. Kozak sequence with the start codon (ACCATG) was added at the 5’-end of the DNA sequence. At both sides of sequence, two restricted enzyme sites (XhoI and BamHI) were predicted. Also, a stop codon (TAG) was added at the 3’-end of target sequences. The final DNA sequences were designed and synthesized. Artificial gene sequences encoding target immunogenic epitopes of E6, E7 and L1 create 397 bp. The scheme of recombinant DNA construct produced in this study has been shown in Figure 1. Validity of synthesized sequences was confirmed using automated sequencing. The construct was subcloned into pIRES and named as pIRES-E6/E7/L1.
2.2. Cells Line, Mice and Media
The TC-1 cells were purchased from Pasteur Institute, Tehran, Iran. The original cells were prepared by transformation of C57BL/6 mouse primary lung cells with the HPV16 E6/E7 oncogenes and human activated H-ras (13). The cells were cultured in Roswell park memorial institute (RPMI) 1640 cell-culture medium (Gibco Invitrogen; Paisley, Scotland, UK), supplemented with 10% fetal bovine serum (FBS) (Gibco BRL), 100 IU/mL penicillin,100 μg/mL streptomycin, 2 mM glutamine, 1 mM sodium pyruvate, and 2 mM nonessential amino acids, at 37°C incubator with 5% CO2.
Female C57BL/6 mice (3 - 4 weeks old) were purchased from the Pasteur institute, Tehran, Iran (n = 25) and were used for experimental purposes. All procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of animal’s laboratory of Tarbiat Modares University. Before the experiment, mice were housed in standard condition with free access of food and water.
2.3. Vaccine Challenge
To evaluate anti-tumor activity of pIRES-E6/E7/L1 and peptide in vivo, TC-1 cancer cells (1 × 106) were subcutaneously (S.C.) injected in the right flank of each mouse. After 5 days, when tumors were palpable (about 100 mm3), the mice were randomly divided into 5 groups of 7 animals each. Tumor size was measured using caliper, and the weight of each mouse was measured by a scale. Tumors were monitored 4 times per week and their volumes were calculated by the equation “Volume = length × width2 / 2”.
The expression vector that contained pIRES-E6/E7/L1 was transformed in E. coli DH5α strain cultured in Luria Broth Medium and purified by the plasmid maxi kit (Gene All, Korea).
Two weeks after TC-1 cells injection, mice were immunized subcutaneously with 100 μL phosphate buffered saline (PBS; negative control), 100 μg naked DNA vaccine encoding pIRES (negative plasmid control) and pIRES-E6/E6/L1 in PBS, on days 0 and 10. Mice were also injected intraperitoneally with a dose of 1 mg/kg /day of peptide. Negative control mice groups were used for elimination of nonspecific responses and other environmental interferences. Finally, animals were sacrificed on day 21 after the final immunization, their spleens were isolated aseptically for in vitro splenocytes culture.
2.4. MTT Proliferation Assay
MTT assay actually determines the metabolic activity of mitochondria and correlates well with the number of viable cells. This assay has been previously used to evaluate endothelial cell proliferation (14). Since the proliferation of immune cells in the spleen represent responses to the immune stimulators, an MTT proliferation assay was used to evaluate the stimulating effect of E6, E7 and L1 proteins on proliferation of spleenocytes. Briefly, cells (300,000/well) were seeded onto 96-well culture plates and were treated with 10 mg/mL of each protein for 72 hours. Then, cells were incubated with MTT yellow dye (5 mg/mL in PBS) at 37°C incubator with 5% CO2 for 3 hours. The purple formazan product was dissolved in DMSO, and the absorbance was measured at 570 nm using an ELISA reader. The MTT assay was done in triplicate.
3. Results
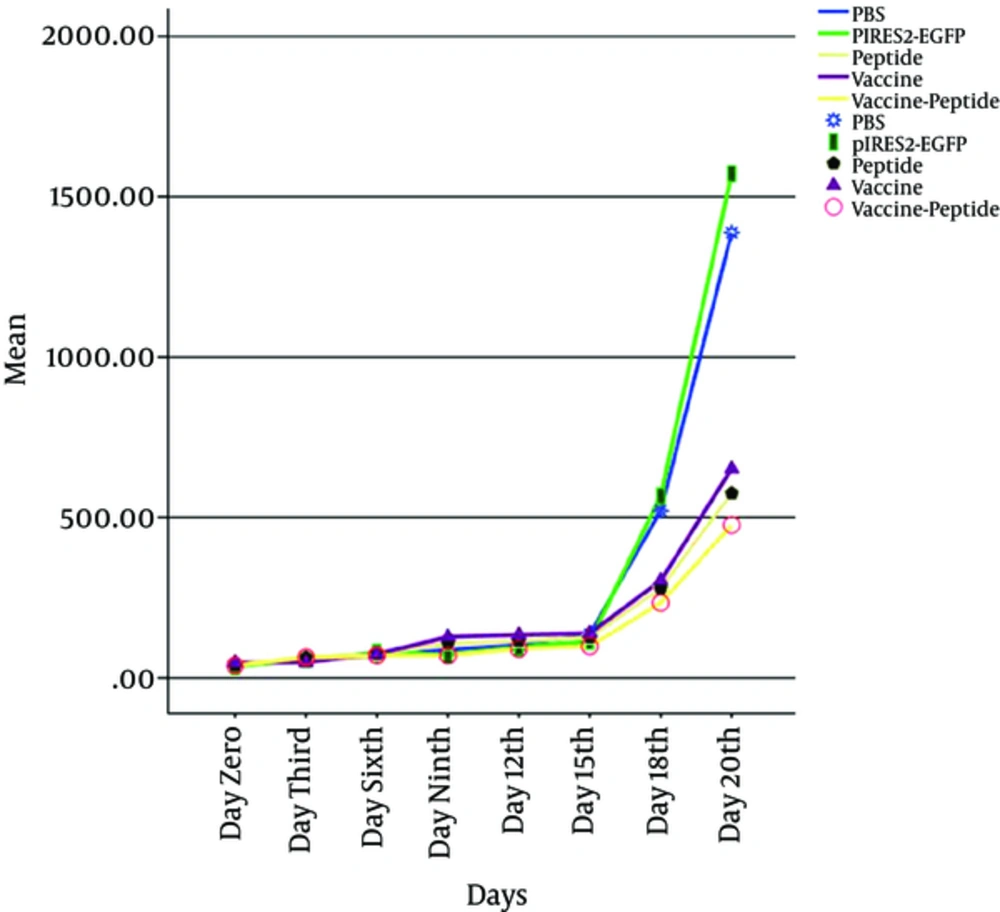
Mice were injected subcutaneously by TC-1 tumor cells, monitored for tumor formation, and the tumor was observed after 5 days post inoculation. Then to investigated therapeutic antitumor effects of vaccine, peptide and vaccine-peptide, the mice were injected by pIRES-E6/E7/L1 and peptide as described above and were followed for tumor development. Evidence of therapeutic effects of vaccine and peptide was investigated at the final days in vaccine and vaccine-peptide groups with significant rate inhibition of tumor growth (P < 0.05). There was no significant difference in tumor size between the test groups (Figure 2).
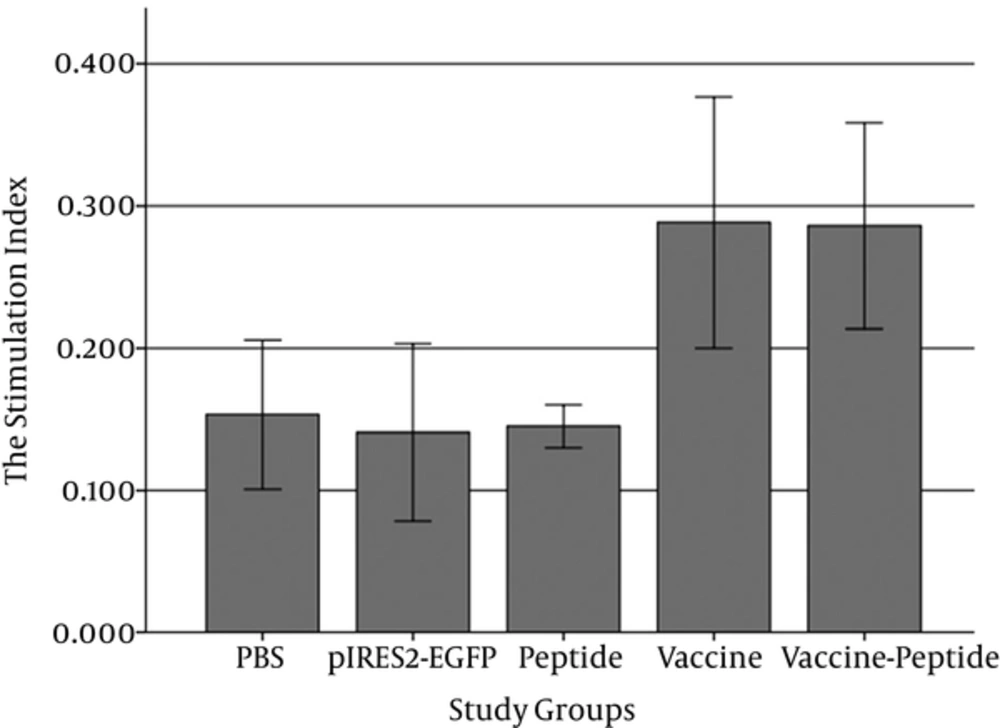
In order to determine how pIRES-E/E7/L1 stimulates spleen’s lymphocytes, MTT assay was performed to evaluate the proliferation of spleenocytes in response to stimulation with the E6 and E7 and L1. So the spleenocytes were extracted and harvested for 48 hours (Figure 3) and formation of formazan crystal was determined by solving the crystals in DMSO. Lymphocyte proliferation in the pIRES-E6/E7/L1 group, as vaccine group, and pIRES-E6/E7/L1-peptide group was significantly higher than in the other groups especially the negative control group (P < 0.05). (Figure 4). As seen in Table 1, the stimulation indexes of these groups were higher than those of the control groups. (Table 1).
After all injections, spleens of individual mice (five/group) were removed and lymphocyte proliferation was evaluated using the MTT method. Formazan crystal formation after incubation with MTT was determined by solving the crystals in DMSO, and the OD was read at 570 nm. Lymphocyte proliferation in the vaccine and vaccine-peptide groups was significantly higher than in the other groups especially the negative control group (P < 0.05).
| Group | The Stimulation Index |
|---|---|
| PBS | 0.153 ± 0.09 |
| pIRES2-EGFP | 0.141 ± 0.07 |
| Peptide | 0.145 ± 0.04 |
| Vaccine | 0.288 ± 0.13 |
| Vaccine-Peptide | 0.286 ± 0.11 |
4. Discussion
Human papilloma virus type 16 (HPV16) is the most common HPV type that is associated with severe cervical dysplasia and cancers (15). Many strategies have been proposed for treatment of cervical cancer and one of them is immunotherapy against the HPV. In recent studies, it has been found that E6/E7/L1 antigens of HPV are suitable candidates to generate DNA vaccines as shown previously (4, 16, 17).
Recently preventive vaccines based on L1 antigens have been produced and have been successful in prophylactics goals, but these vaccines have shown no therapeutic effects and are expensive for many developing countries (18).
As mentioned in previous studies, the expression vector containing of E6/E7/L1 genes were evaluated beneficial (19). In this study we used their cellular epitopes in a single vector and the reduction effect of the construct in tumor size and proliferation of immune cells in the spleenwas shown. Based on our experiment the optimized truncated epitopes of HPV tumor antigens (E6/E7/L1) in mammalian expression vector had therapeutic effects in comparison to the peptide alone.
It seems that the therapeutic effects of DNA vaccine can be tolerated by the amount of inoculated tumor cells and the age of mice, which was similar to other related studies. Diniz et al. showed that therapeutic effects of DNA vaccine can be higher if 5 × 104 cells were injected instead of 5 × 105 cells and it seems that using the older mice is better maybe for having more developed immune systems (20, 21).
Despite the reduction in tumor size in the group that received peptide alone, it was not statistically significant by using 50 µg/kg of the peptide (12). Maybe higher doses response of inoculums or other related differences in susceptibility must be considered in future studies.
4.1. Conclusions
In conclusion, our data suggest that although combination targeted therapies are more preferable strategies, in some circumstances, combination therapies can affect the immune response and need more detailed pilot studies. The dosage and intervals of target therapies and detailed specifications of animal species, sex, age, microbiological status and weight to assess their effect on specific tumor models should be investigated properly.