1. Introduction
Anorectal melanoma (ARM) is a rare and aggressive malignancy. It accounts for less than 0.05% - 4.6% of all anorectal malignancies and 0.4% - 1.6% of all malignant melanomas. ARM is the third most common primary origin of melanoma following skin and retina. Majority of patients present in the 6th and 7th decades. There is a slight female predominance (1). It has a predilection for early infiltration and distant spread resulting in poor overall survival. Because of the rarity of this tumor, it has been difficult to define an optimal treatment approach (2).
2. Case Presentation
A 60-year-old male patient presented with complain of constipation of 7 - 8 months duration, per rectal bleeding for last 6 - 7 months and swelling in bilateral inguinal regions-left sided swelling since last 5 - 6 months and right swelling since 1 1/2 months.
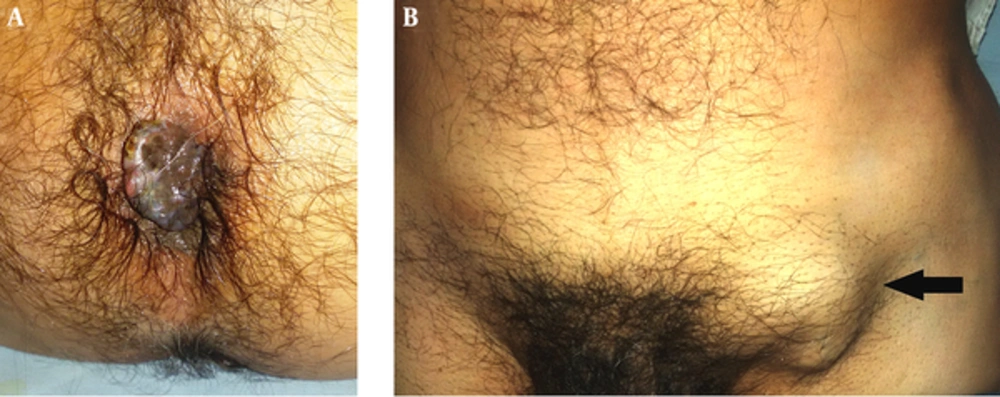
On clinical examination, an oval nodular mass (approximately 3 cm × 2 cm) could be seen protruding from the anal canal, on the right side of anal verge (Figure 1A). Bilateral inguinal region swellings were palpable, which was far more pronounced on the left side (Figure 1B). Per rectal digital examination revealed a firm, exophytic growth in continuity with the visible external mass, extending along the right and anterior walls of the anal canal and rectum. The cranial margin of this mass could not be reached. Examining finger revealed slight bleeding.
Proctoscopy revealed a haemorrhoid-like pigmented lesion. Biopsy taken from this anorectal growth showed malignant melanoma and fine needle aspiration cytology (FNAC) from the left inguinal swelling gave the impression of metastatic malignant melanoma.
Complete blood counts, liver function tests, renal function tests of the patient showed values within normal range. The patient was negative for human immuno virus (HIV), Hepatitis B and C (HBsAg and HCV). Carcino embryonic antigen (1.08 ng/mL) was also in normal range. Chest X Ray was normal. The patient had no neurological symptoms. He had no complaint of any bone pain. Skin and ophthalmological examination revealed no abnormalities.
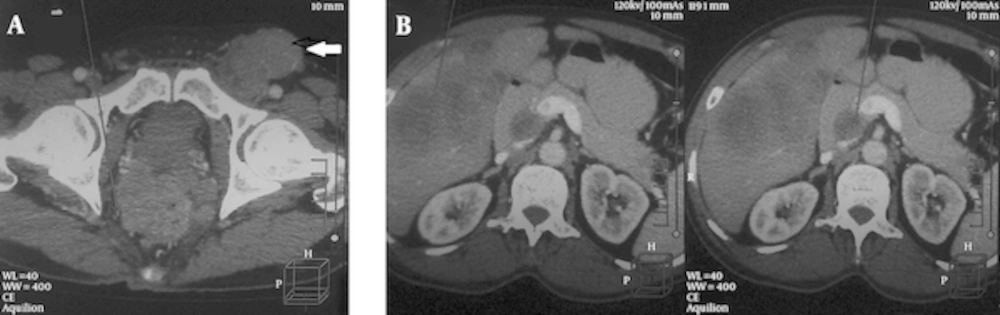
Computer tomography (CT) scan abdomen (Figure 2A) showed circumferential polypoidal mural thickening with heterogeneous moderately enhancing mass in anorectal region with exophytic component in right lateral wall in close abutment with prostatic parenchyma posteriorly (possible infiltration). Approximate length of involved segment was 14 cm from anal verge. Thickness was 2.6 cm at anal verge and 1 cm in the rectum. Exophytic component measured 6.7 × 6.6 × 5.6 cm. Mesorectal fascia showed multiple lymph nodes and possible infiltration.
Enlarged lymph nodes were seen in Left Iliac region, Left para-aortic, pancreatico-duodenal groove and few mesenteric implants in the sub-hepatic region. Large conglomerate lymph node mass was also seen in Left Inguinal region (4.3 × 5.1 × 4.6 cm). Liver was mildly enlarged and showed multiple metastases, the largest lesion being 6.5 × 8.0 × 7.5 cm (Figure 2B). Spleen parenchyma also showed possibly metastatic lesion. Whole body positron emission tomography computed tomography (PET-CT) could not be done due to financial constraints.
Dacarbazine (1000 mg/m2 per cycle every three weeks) was prescribed to patient. After three cycles of chemotherapy, although the patient reported improvement in bleeding, clinical and radiological examination did not reveal a significant disease response. The patient did not turn up for 2 months. A telephone call was made to his son, who informed us that the patient had expired, 4 1/2 months after biopsy diagnosis of the disease.
3. Discussion
Malignant melanomas (both cutaneous and mucosal), result from malignant transformation of melanocytes, which are cells derived from the embryologic neural crest. During fetal development, these cells migrate to various sites of the body, mainly to the skin. However, melanocytes may also reside in the eyes (retina and uveal tract) and mucosal surfaces (head and neck, anorectum, female genitalia). Anorectal melanoma (ARM) is an aggressive malignancy and patients usually present with bleeding, mass, constipation, diarrhea or asymptomatic cases during medical check-up (3). Weinstock (4) reported that at the time of diagnosis only 37% of ARM is confined at the anorectal area, 41% has regional spread and 22% has distant metastasis. Lymphatic spread is common and tends to involve mesenteric and inguinal lymph nodes (5). The major sites of distant metastasis are brain, lung, liver, and bone (6). Our patient had inguinal and abdmino-pelvic nodal disease: and liver and spleen as metastatic sites at presentation.
The prognosis of ARM is very poor with five year survival rate of 6% - 22%, and the median survival of 19 - 26.4 months (7). If ARM is local confined, the five year survival rate is 37% - 50%. However, with regional and distant metastasis, the five years survival rate decreases to 7% - 17% and 0% - 6%, respectively (2, 4).
Possible reasons for the poor prognosis include vague symptoms causing delay in diagnosis, rich lympho-vascular network allowing early metastasis, intrinsic aggressive biologic behavior and a tendency towards chemotherapy resistance (8). Our patient was initially getting treatment for hemorrhoids at the local dispensary and presented to us late, at a time when he had developed extensive loco-regional disease and distant metastasis.
For localized ARM surgical therapy is indicated. Most series report similar survival in patients treated by wide local excision or abdominoperineal resection (APR) although the latter has proved more effective to control the local disease but, without clear improvement in survival (9).
No systemic therapy regimen for metastatic anal melanoma is considered standard of care. Treatment has been based on drugs developed for advanced cutaneous melanoma and included Cisplatin, Vinblastine, Dacarbazine, INF, and interleukin-2 (10). Dacarbazine has been the most commonly used therapy for metastatic melanoma. Response to dacarbazine has generally ranged from approximately 10% to 20%. Compared with dacarbazine alone, no single agent or combination of agents has yielded a significant improvement in durable or complete responses or in overall survival in a randomized trial prior to approval of newer agents (11).
Newer therapeutic agents to have been incorporated in the management of disseminated melanoma are Ipilimumab (monoclonal antibody directed against CTL4-A) which has shown better overall survival in phase III study comparing Ipilimumab and Dacarbazine versus Dacarbazine alone (12); BRAF inhibitors like Vemurafenib (13), Dabrafenib (14) and KIT inhibitors like Imatinib (15).
3.1. Conclusion
Anorectal melanoma is a disease with grave prognosis especially when the patient presents with distant metastasis. The best hope for improved survival lies in early detection and complete surgical removal. Since the complaints are usually nonspecific and symptoms are vague, a high index of suspicion and subsequent clinical work up is vital to detect the disease at an early stage.