1. Background
Chronic lymphocytic leukemia (CLL) is characterized by the gradual accumulation of CD19-CD5-malignant B cells along with immune cell dysfunction (1). It has been reported that immune response and homeostatic control defects in the T cell and the leukemic B cell compartments (2). Abnormalities in these compartments may contribute to the inability of the immune system to recognize and destroy the leukemic cells (3-6).
CLL is the second most common type of leukemia in adults (7, 8). It often occurs during or after middle age, and is rare in children (9). Usually CLL does not cause any symptoms and clinical course of disease is variable (9). Some of these patients have a stable situation (even to the end) and do not require treatment, while others have progressed disease despite treatment (10). So, evaluation of new diagnostic and therapeutic strategies for B-CLL seems necessary and attractive. Prognosis of disease mainly depends on the clinical and laboratory outcomes of disease. However, many patients are asymptomatic at the time of diagnosis.
Adenosine deaminase (ADA) (EC 3.5.4.4) is a hydrolytic enzyme that involves in the deamination of adenosine and deoxyadenosine nucleosides, forming inosine and deoxyinosine, respectively (11). ADA is widely distributed in human tissues and is involved in immune system development (12, 13). In particular, ADA has an important role in proliferation and differentiation of lymphoid cells and plays a major role in various stages of lymphocyte maturation (14). ADA is also the main regulator of adenosine concentration in plasma which is involved in development of inflammatory response and cytokine production (15).
Alteration in serum ADA activity has been reported in a broad range of diseases such as tuberculosis, HIV, lung cancer, chronic heart failure and chronic obstructive pulmonary disease (16-20). ADA is known as a marker of T-lymphocyte activation (21). Furthermore, it has been shown in several studies that ADA activity may be useful both in the diagnosis and monitoring of some malignancies (21, 22).
Considerably, CLL is one of the most common lymphomas in Iran (23). In addition, the current tests for diagnosis and follow up of CLL patients are difficult, slow and expensive. ADA is measured calorimetrically and coefficient of variation (%CV) for this method is about 3%; it has a high reproducibility and very inexpensive materials used for this method (16).
2. Objectives
Because of simplicity, rapid use and low priced of ADA test, in present study, we measured total ADA, activity in serum of patients with B-CLL and healthy subjects to assess their diagnostic validity as a marker for diagnosis of CLL.
3. Materials and Methods
3.1. Patient Selection
All 87 B-CLL patients (68 males and 19 females with an average age of 66.27 ± 10.68 years) who admitted to the Sanandaj Tohid hospital (Kurdistan, Iran) during a period of January 2012 until April 2014 were enrolled in this case-control study. Diagnosis of disease was based on clinical and laboratory characteristics, including hematologic and immunologic data. The youngest patient was 44 years old and the oldest one was 91 years. Based on Rai staging system (24), staging of the patients were done. The control group consisted of 100 healthy individuals (50 women and 50 men) with a mean age of 53.84 ± 8.11 years, with non-malignancy (negative pathological tests). Written informed consent was obtained from all patients and the study was approved by the ethics committee of Kurdistan University of Medical Sciences. Criteria for inclusion of individuals and treatment conditions were determined previously (25, 26). Of all cases, 22 patients were treated with FC (fludarabine with cyclophosphamide) and CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone).
3.2. Measurement of ADA Activity
the fasting blood serum samples were collected and used to measure the enzyme activity. Adenosine was obtained from Sigma-Aldrich (Saint Louis, Missouri 63103, USA). Sodium di-hydrogen phosphate [NaH2PO4.H2O], di-sodium hydrogen phosphate [Na2HPO4, 12H2O], Ammonium sulfate [(NH4) 2SO4], Phenol [C6H5OH], Sodium nitroprusside [Na2 (Fe(CN)5NO)], soda [NaOH] were obtained from (Merck Chemicals Ltd, Germany). Serum total ADA activities were determined by Giusti method (27). Briefly, duplicate 25 μL samples were incubated for 60 minutes at 37°C with 500 μL of 21 mM adenosine (one sample) in 50 mM phosphate buffer, and the released ammonia was determined by its reaction with 1.5 mL phenol nitroprusside in 30 minutes (106 mM phenol plus 0.17 mM sodium nitroprusside) in the presence of 1.5 mL of sodium hypochlorite (11 mM NaOCl plus 125 mM NaOH), using a spectrophotometer absorption read at 630 nm. To control the presence of ammonium before addition of exogenous adenosine, untreated samples were run in parallel. The results were expressed in U/L.
3.3. Statistical Analysis
Data analysis was carried out using the Kruskall-Wallis and two-way ANOVA tests. A P value < 0.05 was considered statistically significant. Spearman rank coefficients were used to express the correlation between variables. The significance of the correlation was denoted by a P value < 0.05. All statistical analyses were done using software SPSS version 16. Receiver operating characteristic (ROC) curves were constructed to establish a sensitivity-specificity relationship. Cut-off values that provided the best combination of sensitivity and specificity were determined by ROC curve analysis. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+), negative likelihood ratio (LR-) and accuracy were calculated (20).
4. Results
The means and standard deviations of ADA for B-CLL and healthy groups were 51.67 ± 20.99 U/L and 19.03 ± 6.17 U/L, respectively (P < 0.0001). Table 1 represents the ADA activity in patient group according to the stage of disease. As shown in this there there were not any significant differences between groups in regard to stage.
Abbreviations: ADA, adenosine deaminase; U/L, units per liter of enzyme activity.
aValues are expressed as mean ± SD.
bSignificant difference (P < 0.05).
Clinical and laboratory outcomes of study subjects are shown in Table 2. Our results show that the serum Beta-2-microglobulin (B2M) and Lactate dehydrogenase (LDH) were higher in the patient group than healthy subjects. In addition, the white blood cell (WBC) and red blood cell (RBC) count and Erythrocyte sedimentation rate (ESR) had similar patterns and were significantly higher in patients. Furthermore, platelet (Plt) count was significantly lower in patients compared to healthy group. However, we could not find any differences for B2M, WBC, RBC, LDH, ESR and Plt in various stages. In addition, treatment with chemotherapeutic agents significantly (P < 0.05) decreased the ADA activity in patient group (ADA activity before treatment was 56.55 ± 22.61 U/L while as ADA activity after treatment was 39.38 ± 18.87).
We also determined the possible correlation between ADA activity and laboratory markers in B-CLL group. Our finding demonstrated that there is a significant positive correlation between ADA activity and WBC, LDH, B2M and ESR (Table 3). On the other hand, RBC and Plt counts had no correlation with ADA activity (Data not shown).
| Stage 0 | Stage I | Stage II | Stage III | Stage IV | |
|---|---|---|---|---|---|
| B2M, mg/L | 2.90 ± 1.43 | 4.29 ± 1.49 | 4.87 ± 2.55 | 4.27 ± 1.71 | 5.56 ± 2.03 |
| Total | 4.71 ± 2.04 | ||||
| LDH, U/L | 375.17 ± 101.49 | 360.67 ± 73.78 | 388.86 ± 11.52 | 315.67 ± 11.87 | 382.89 ± 141.97 |
| Total | 369.96 ± 116.03 | ||||
| ESR, mm/h | 12.07 ± 7.97 | 3.96 ± 1.83 | 11.86 ± 9.89 | 7.38 ± 5.96 | 9.02 ± 7.61 |
| Total | 8.65 ± 7.38 | ||||
| Plt Count, × 105/µL | 1.84 ± 0.45 | 1.84 ± 0.56 | 1.96 ± 0.66 | 1.92 ± 0.56 | 1.27 ± 0.44 |
| Total | 1.64 ± 0.58 | ||||
| WBC Count, × 103cell/µL | 59.40 ± 42.65 | 85.54 ± 10.33 | 56.20 ± 44.26 | 61.00 ± 46.61 | 17.47 ± 23.01 |
| Total | 47.18 ± 58.84 | ||||
| RBC Count, × 106cell/µL | 12.85 ± 1.30 | 12.67 ± 2.46 | 13.64 ± 1.64 | 11.33 ± 0.78 | 12.41 ± 1.46 |
| Total | 12.56 ± 1.70 | ||||
Abbreviations: B2M, beta-2-microglobulin; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; Plt, platelet; RBC, red blood cell; WBC, white blood cell.
| Pearson Correlation | P Value | |
|---|---|---|
| WBC | 0.49 | < 0.05 |
| LDH | 0.446 | < 0.05 |
| B2M | 0.621 | < 0.01 |
| ESR | 0.373 | < 0.01 |
Abbreviations: B2M, beta-2-microglobulin; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; WBC, white blood cell.
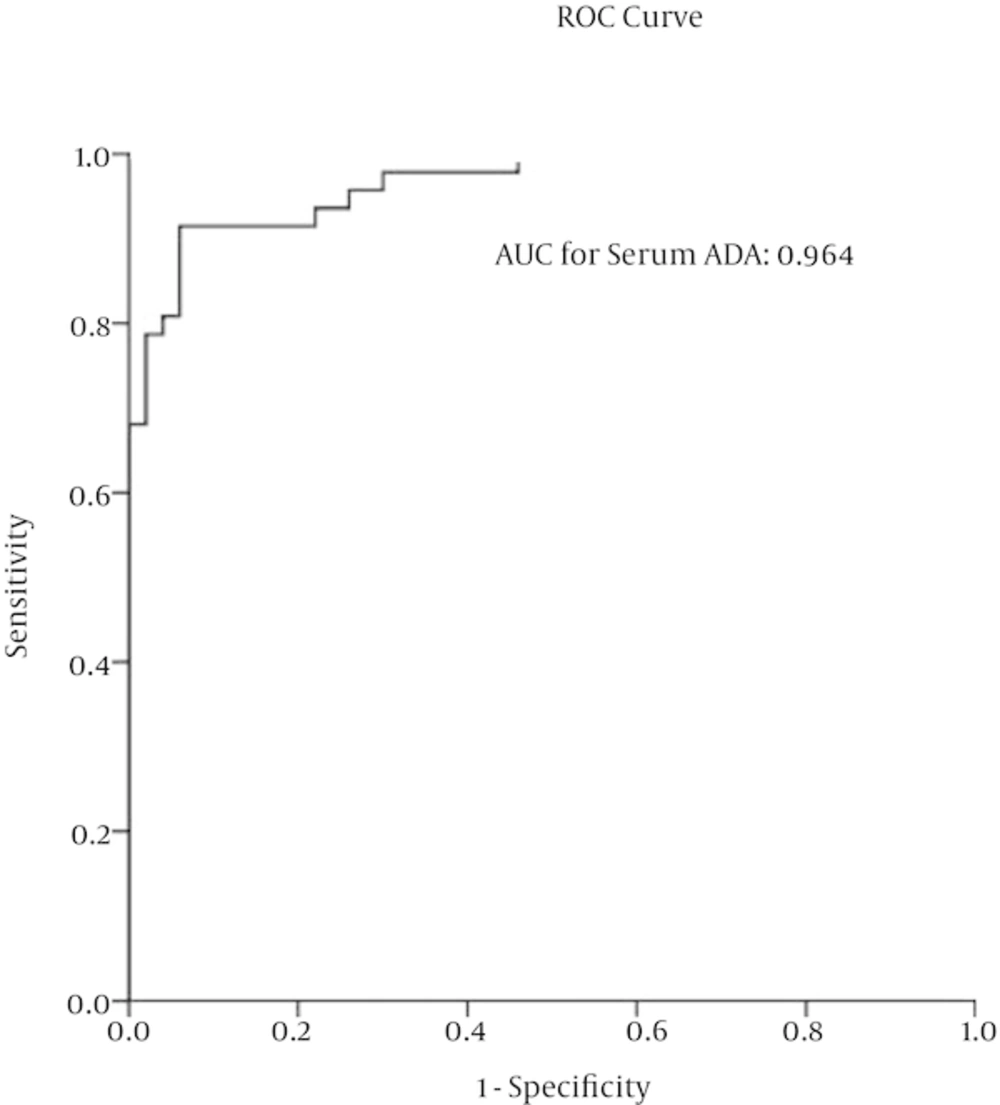
ROC curve analysis revealed that cut-off level is 27.97 U/L for enzyme activity in serum sample. Using this cut-off level for serum ADA activity, sensitivity and specificity were 91% (95%CI = 0.8 - 0.98) and 94% (95%CI = 0.83 - 0.99), respectively. Besides, according to obtained sensitivity and specificity, positive predictive value, negative predictive value, positive likelihood ratio and negative likelihood ratio were evaluated (Table 4 and Figure 1).
| Cut-Off, U/L | Sensitivity | Specificity | LR+ | LR- | PPV | NPV | Accuracy, % | |
|---|---|---|---|---|---|---|---|---|
| Values | 27.97 | 0.91 | 0.94 | 15.17 | 0.1 | 0.91 | 0.92 | 93 |
Abbreviations: LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
5. Discussion
Several investigations showed the role of adenosine deaminase in immune system development. It seems that ADA has an important role in maturation of lymphocytes and high ADA activity is seen in immature lymphocytes (28, 29). In addition, there are several studies that represent high ADA activity in various malignancies. Patients with lung cancer, breast cancer, ovarian cancer, gastric carcinoma and colorectal cancer have increased level of serum ADA activity (21, 30-33). Our results also demonstrated higher enzyme activity in B-CLL patients compared to healthy subjects.
In addition, our results revealed that there is a direct correlation between B2M, WBC, LDH and ESR with serum ADA activity. These markers, especially B2M could be used to evaluate the severity and spread (stage) of multiple myeloma, to help determine the prognosis of cancers such as multiple myeloma and lymphoma, and may sometimes be ordered to evaluate disease activity and the effectiveness of treatment (34). However, in our study we could not find any correlation between these markers and the stage of disease. It may be because of the low number of patients dispersed in various stages. Furthermore, the direct significant correlation of ADA with these markers that could be seen in our study suggests the serum ADA activity as a good prognostic tool in CLL.
There are a few studies that determined ADA activity in lymphocytes (35-38). The results from these studies are completely contradicted to our results. They suggest that ADA activity in malignant lymphocytes is lower than normal ones. However, previous studies clearly showed that although the location of the enzyme is mainly cytosolic, ADA has been found in membrane fractions (39) and realization of ADA to blood stream may rise by increasing the cell numbers. To our knowledge, our study is the first investigation that evaluated the serum ADA activity with clinical outcomes in B-CLL patients. Our results revealed that serum ADA activity in B-CLL patients is significantly higher than controls. Furthermore, we showed that if we use a cut-off value equal to 27.97 U/L, the sensitivity and specificity of serum ADA test is 91% and 94%, respectively.
Besides, our previous studies showed that ADA activity tangibly depends on the wavelength used for measurement of ADA. In addition, it seems that it has a wide range of results in different studies. It was suggested that this alterations could be due to sampling and/or patient selection and analysis methods. Furthermore, reference intervals for healthy subjects that have been reported in different ethnic groups are very diverse (16).
In summary, we showed that serum total ADA activity in B-CLL subjects is significantly higher than controls. In addition, our results also showed a direct correlation between WBC, LDH, B2M and ESR with ADA activity. Furthermore, we showed that serum ADA activity is a good test for differentiating B-CLL patients from healthy subjects and it may be used as an alternative or additional test along with the other tests for diagnosis and/or prognosis of B-CLL.
It can be concluded that determination of serum ADA activity might be a simple, rapid, and inexpensive diagnostic tool for screening of B-CLL patients compared to current costly, laborious, and time consuming markers.