1. Background
Lactobacilli are a group of probiotic bacteria which colonize the human gastrointestinal tract as well as female urogenital tract (1). Several studies have shown tumor-suppressing properties for certain lactobacillus strains (2, 3). Such anti tumor effects have been postulated to be exerted via different mechanisms such as inhibition of pathogens colonization (4), induction of immune system (5, 6), direct cytotoxic effects on cancer cells (2, 3), antimutagenic effects (7) as well as modulation of carcinogens metabolism and prevention of DNA from oxidative damage (8).
MDA-MB-231 is an aggressive and highly metastatic cell line originated from a high grade tumor (9, 10). HeLa is a cervical cancer cell line in which integration of the human papilloma virus type 18 (HPV-18) genome is proposed as a initiator event in the tumorigenesis (11). Previously we have demonstrated that treatment with lactobacilli culture supernatants decreases the expression of HPV E6 oncogene so it may be of therapeutic value (12). HT-29 is a colorectal adenocarcinoma cell line in which the apoptotic effects of different lactobacilli strains have been evaluated (13).
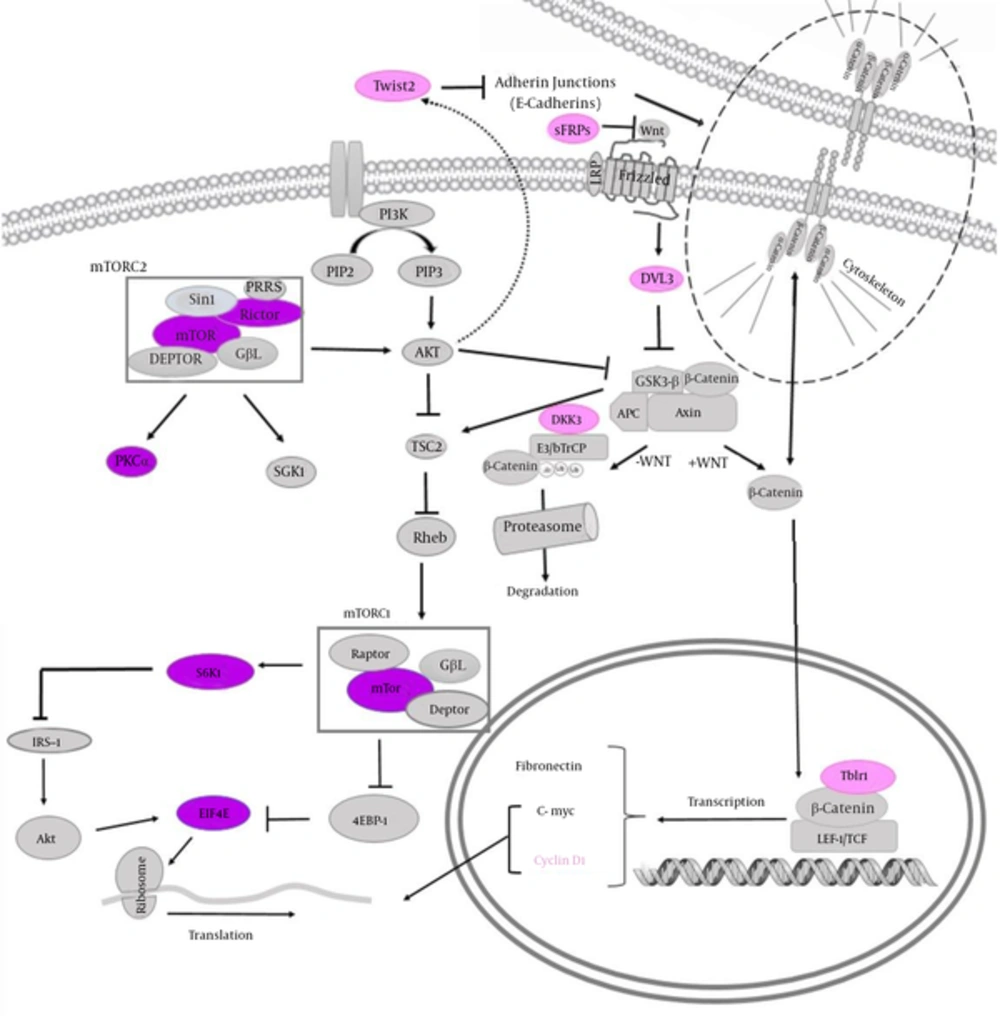
The phosphoinositide 3 kinase (PI3K)/Akt/mammalian (or mechanistic) target of rapamycin (mTOR) pathway is an essential pathway leading to cell growth and tumor proliferation. This pathway is involved in resistance to endocrine therapy, HER2-directed therapy and cytotoxic therapy in breast cancer (14). In addition, this pathway has been shown to be frequently deregulated in cervical cancers (15) as well as colorectal cancers (16). Activation of mTOR complex 1 leads to phosphorylation of some factors and results in selective overexpression of cyclin D1, Bcl-2, Bcl-xL and vascular endothelial growth factor (VEGF) as well as the nucleocytoplasmic transport of selected mRNA such as cyclin D1. Consequently, it increases cell proliferation, survival, and angiogenesis. S6K1 is a critical regulator of cell growth, which phosphorylates ribosomal protein S6 and other important targets. Both eIF4E and S6K1 are involved in cellular transformation, and their overexpression has associated with poor cancer prognosis. mTOR and RICTOR are implicated in AKT phosphorylation and activation and have role in AKT interaction with the apoptosis regulator BAD. mTOR complex 2 has been involved in the posphorylation of PRKCA (17) which is in turn implicated in various cellular processes such as cell adhesion, cell transformation and cell cycle checkpoint. PRKCA has been associated with metastatic potential of breast cancer through the activation of matrix metaloproteinases and has been regarded as poor prognostic marker as well as a therapeutic target in cancer patients (18, 19).
Deregulation of the canonical Wnt/β-catenin signaling pathway has been implicated in several cancers including breast, cervical and colorectal cancers (20). SFRP2 encodes a soluble modulator of Wnt signaling. Methylation of this gene is a potential marker for the presence of colorectal cancer (21), cervical cancer (22) as well as breast cancer (23). Similar to other secreted Frizzled related proteins, SFRP2 acts as antagonist of Wnt pathway by squelching Wnt ligands (24). DKK3 codes for a Dickkopf (Dkk) protein which effectively inhibits Wnt signaling by preventing Wnt interaction with LRPs (24). DVL3 codes one of Disheveled (Dvl) proteins which becomes phosphorylated upon Wnt stimulation thereby stabilizes β-catenin (24). TWIST genes code for an essential factor for epithelial-mesenchymal transition (EMT). The key TWIST isoform which couples aberrant signals from EMT to senescence has been shown to be TWIST2. This isoform has been suggested as an important candidate biomarker for cervical cancer prognosis (25). TBLR1 has a critical role in nuclear β-catenin function. In addition, the depletion of TBL1-TBLR1 has considerably hindered Wnt-beta-catenin-induced gene expression and oncogenic growth in vitro and in vivo (26). Finally, CCND1 codes for cyclin D1, an oncogene and an important positive regulator of the G1/S phase which is a downstream target of β-catenin (27). Figure 1 shows the position of selected proteins in the mTOR and Wnt pathways as well as the interaction of these pathways.
2. Objectives
In this study, we aimed at determination of cellular pathways involved in the cytotoxic effects of two lactobacilli strains namely, L. rhamnosus and L. crispatus against MDA-MB-231, HeLa and HT-29 cancer cell lines.
3. Materials and Methods
3.1. Selection of Genes From mTOR and Wnt/β-Catenin Pathways
Based on previous expression studies, 5 genes were selected from mTOR pathway to evaluate expression of different parts of this pathway: RICTOR, S6K1, EIF4E, PRKCA and MTOR. Furthermore, SFRP2, TBLR1, DVL3, CCND1, DKK3 and TWIST2 genes were chosen from Wnt/β-catenin pathway to evaluate their expression following treatment with lactobacilli supernatants. Selection of genes from this pathway was based on previous reports regarding epigenetic deregulation in various cancer types. Fold changes in the expression of these genes have been analyzed after certain treatments.
3.2. Cell culture
This study has been approved by the ethical committee of Shahid Beheshti University of Medical Sciences. Human cervical cancers (HeLa), breast cancer (MDA-MB-231), colorectal cancer (HT-29) as well as human lung fibroblst (MRC5) cell lines cell lines were purchased from the Pasteur Institute, National Cell Bank of Iran and cultured according to previous studies (28).
3.3. Preparation of Supernatants From Lactobacillus Cultures
Microaerophilic conditions were applied for culture of L. crispatus strain SJ-3C-US and L. rhamnosus strain GG in de Man Rogosa Sharpe (MRS) broth (Merck; pH 6.5) as described in previous publications (28). To check the probable effect of lactobacilli-produced lactic acid on cell cultures, the pH of MRS controls have been adjusted by lactic acid based on the corresponding lactobacilli supernatant pH. In brief, the experiments comprised L. crispatus supernatant, pH 4.3 (LCS); L. rhamnosus supernatant, pH 4.05 (LRS); MRS, pH 6.5 and MRS adjusted with lactate (MRL) pH 4.05 or 4.3.
3.4. MTT Assay
Cell growth inhibition was computed by MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay kit (Sigma, St. Louis , MO) according to previous studies (29). Overnight treatments with lactobacilli culture supernatants cells performed with different concentrations from 10% to 100% (v/v). Plates were incubated at 37°C under 5% (v/v) CO2. Cell viability was computed using the following equation (Equation 1):

3.5. RNA Isolation, cDNA Synthesis and Quantitative RT-PCR (qRT-PCR)
The AccuZolTM total RNA extraction solution (Bioneer, Korea) was used to isolate total RNA from cultured cells. Nanodrop 2000 c spectrophotometer (Thermo Scientific) was used for determination of RNA concentration. Changes in mRNA expression of desired genes were analyzed by quantitative PCR (qPCR) after reverse transcription of 1 μg RNA from each sample with the PrimeScript RT reagent kit (Takara Bio, Ohtsu, Japan). mRNA quantification of genes was implemented in a rotor gene 3000 corbette detection system using SYBR Premix Ex Taq (Takara Bio, Ohtsu, Japan). Primer sequences are listed in Table 1. PCR condition was as follows: an initial denaturation at 95°C for 1 minute, and 40 cycles at 95°C for 15 seconds and 65°C for 1 minute. The final PCR reaction consisted 10 mL SYBR Green master mix, 2 mL cDNA, 0.5 mL each forward and reverse primer (10 pmol) and 7 mL nuclease-free water. Experiments were performed in duplicate for each data point. B2ACTIN mRNA was amplified as a normalizer, and fold changes in each target mRNA expression relative to B2ACTIN were calculated. Melting curve analysis was used to validate whether primers yielded a single PCR product.
| Primer | Sequence | Product Size, bp |
|---|---|---|
| Β2ACTIN | 105 | |
| Forward | AGATGAGTATGCCTGCCGTG | |
| Reverse | GCGGCATCTTCAAACCTCCA | |
| SFRP2 | 162 | |
| Forward | ACCGAGGAAGCTCCAAAGGT | |
| Reverse | GCTCTTGGTCTCCAGGATGATT | |
| TBLR1 | 141 | |
| Forward | GGGAGGAGAATGGAGCACAT | |
| Reverse | CAGGGTTCCAGGCACAGATA | |
| DVL3 | 130 | |
| Forward | TGGACGACGATTTCGGAGTG | |
| Reverse | TTATCAGCACAGAAGGGGGC | |
| CCND1 | 180 | |
| Forward | GAGGCGGAGGAGAACAAACA | |
| Reverse | GAGGCGGTAGTAGGACAGGA | |
| DKK3 | 112 | |
| Forward | CCTGGCAAACTTACCTCCC | |
| Reverse | AGTCTGGTTGTTGGTTATCTTGT | |
| TWIST2 | 142 | |
| Forward | GTGACATCGGACAGAAGA | |
| Reverse | CAAACATAAGACCCAGAAGAAA | |
| RICTOR | 195 | |
| Forward | ACAACAGAGCAACGAGGTA | |
| Reverse | TCTGGATTCTGAAGTGCTAGTT | |
| S6K1 | 167 | |
| Forward | TGCTTAATCACCAAGGTCAT | |
| Reverse | TCCCAAACTCCACCAATC | |
| EIF4E | 159 | |
| Forward | CCAGGGCCAAACGGACATA | |
| Reverse | GGGATTAGGAGTAGGGGTGGT | |
| PRKCA | 131 | |
| Forward | TGCAAAGGACTGATGACCAAAC | |
| Reverse | GGCTGGATCTCCCTGTTCTC | |
| MTOR | 168 | |
| Forward | TGGGGACTGCTTTGAGGTTG | |
| Reverse | ACACTGTCCTTGTGCTCTCG |
Sequence of Primers Used in This Study
3.6. Statistical Analysis
Relative expression software tool (REST©) was applied for comparison of the total expression ratio of the genes between treated and control cells using a randomization test.
Mann-Whitney test was used for comparison of IC50 (concentration giving half-maximal inhibition) of cells treated with lactobacilli culture supernatants and pH- and lactate adjusted as well as pretreated controls in SPSS software (version 16.0). All data were expressed as a mean ± SE of three separate experiments. P < 0.05 was considered as statistically significant.
4. Results
4.1. The Effects of LCS and LRS on HeLa, MDA-MB-231 and HT-29 Cell Proliferation
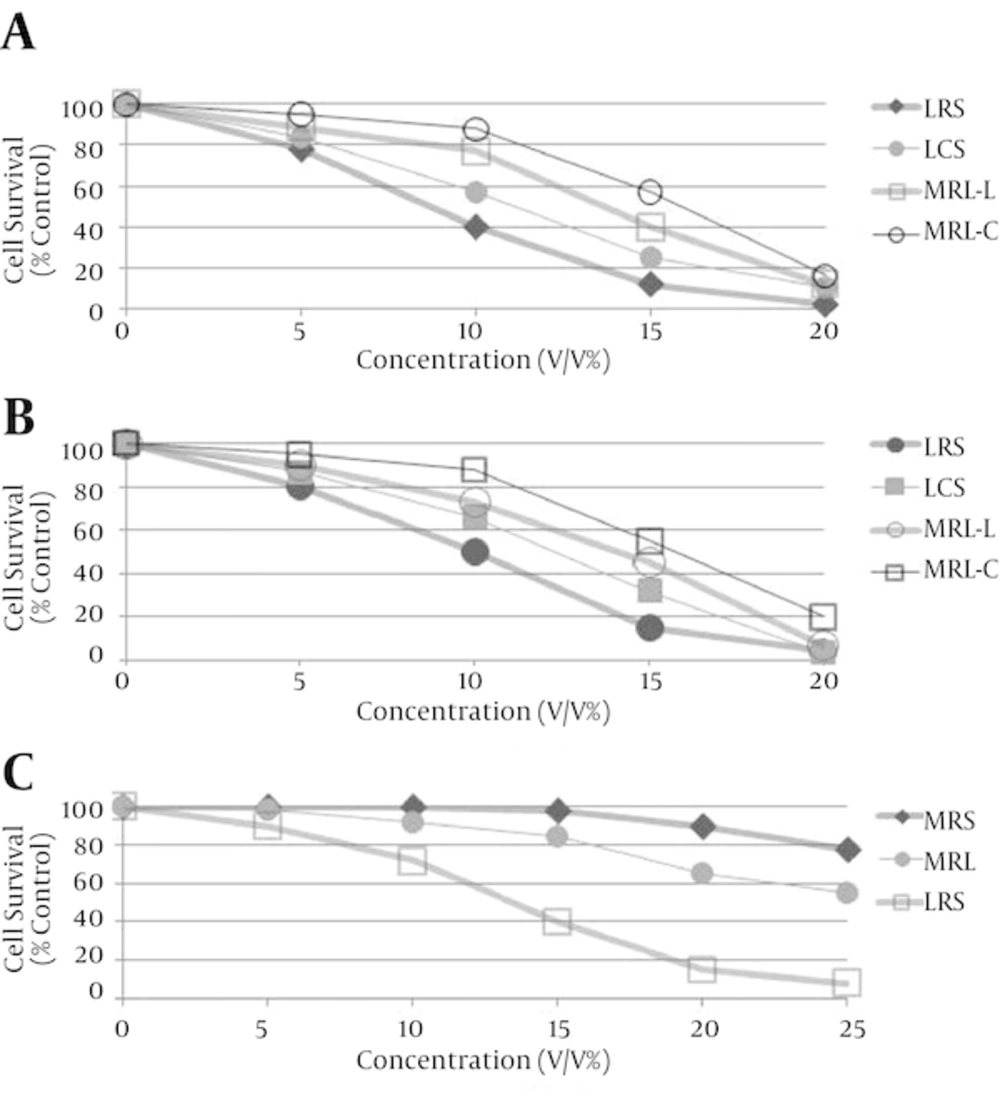
LCS and LRS had no toxicity against MRC5 cells as we reported previously (30). The IC50 values of LRS against HeLa and MDA-MB-231 cells were 9 and 10% (v/v) and those of LCS were 11 and 13% (v/v) respectively. The cytotoxic effects of LCS and LRS against HeLa and MDA-MB-231 cells were higher than those of MRS and MRL (MRS with pH adjusted to that of LCS and LRS) (P < 0.05) (Figures 2A and 2B). LRS but not LCS has cytotoxic effects against HT-29 cell (Figure 2C). The IC50 of LRS against HT-29 cells was 14% (v/v). These results showed that the main cause of cancer cell death was not the acidity. This cytotoxicity against cancer cells can be attributed to a substance other than lactate in the supernatant of the lactobacilli. In addition, cytotoxicity effect of LRS was significantly higher than LCS in three cancer cell lines examined (P < 0.01).
4.2. The Effects of LCS and LRS on Expression of mTOR and Wnt/ β-Catenin Pathways Genes
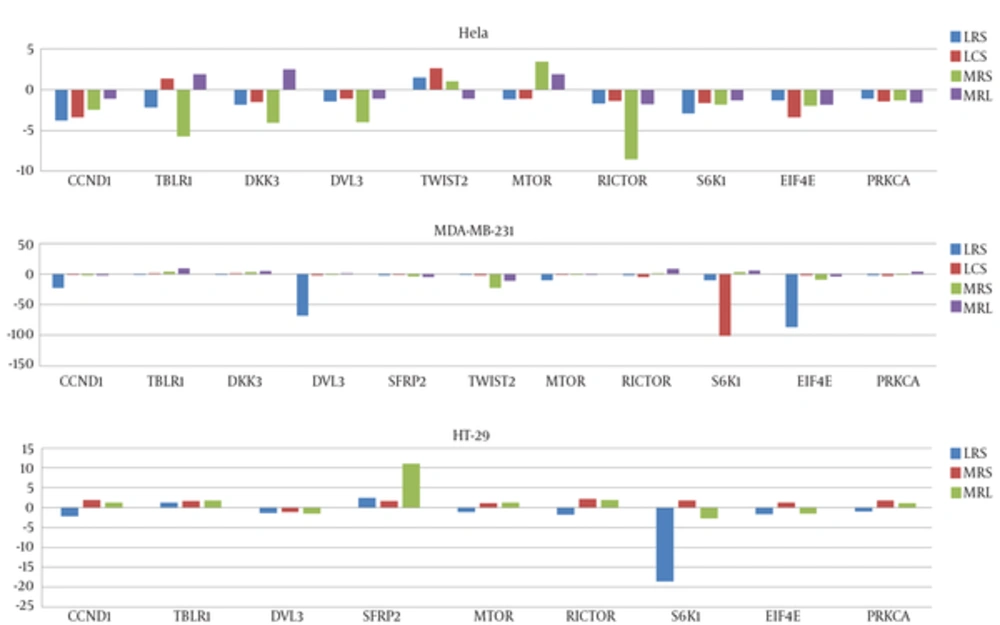
mRNA levels of mentioned genes in cancer cell lines were measured by qRT-PCR before and after treatment with LCS and LRS. After 4 hours treatment of cancer cells with certain percentages (v/v) of culture supernatants (based on the observed IC50 for each LS against a cell line), the effects of LRS and MRS on genes expression was compared with MRS and MRL. All genes have been expressed in three cell line before treatment except for SFRP2 whose expression has not been detected in HeLa cells before treatment but has been significantly upregulated following both LRS and LCS treatments. Figure 3 shows the effects of LCS and LRS on the expression of mTOR and Wnt/ β-catenin pathways genes in three cell lines respectively.
5. Discussion
In this experiment, we have demonstrated downregulation of some mTOR related genes following LS treatment in MDA-MB-231, HeLa and HT-29 cell lines. Of note, the effects of LS on expression of genes have been strain specific as well as cell line specific. For instance, expression of EIF4E has been decreased in MDA-MB-231 cells following LRS treatment by the factors 87. However, LCS has resulted in a 100 fold reduction in S6K1 expression in the same cell line. mTOR inhibitors have shown anti-tumor activity against various human cancers. Combinations of mTOR inhibitors with other treatment strategies such as cytotoxic chemotherapy as well as a variety of targeted molecular agents have shown promising results in many patients (15). Among mTOR inhibitors which are currently in clinical use are rapamycin and its analogs. These drugs have been shown to bind to a domain rather than the catalytic site and inhibit various mTOR functions. A potential drawback of these drugs is that they may activate an mTOR-dependent survival pathway resulting in treatment failure. However, small molecules that compete with ATP in the catalytic site have been shown to inhibit all of the kinase-dependent functions of mTOR without activating the survival pathway (31). Here we have shown that supernatants from two lactobacilli cultures significantly downregulates expression of some genes in mTOR pathway and can be regarded as a mechanism by which these lactobacilli exert their cytotoxic effects against cancer cells. As we have shown the cytotoxic effects of these lactobacilli on cancer cells, the possibility of activating the survival pathway by these lactobacilli is probably ruled out. In addition, considering the role of mTOR pathway in resistance to target specific therapies in breast cancer, downregulation of some mTOR genes in triple negative MDA-MB-231 cells by lactobacilli may be of therapeutic value.
In order to translate the result of these kinds of studies into the clinical use, it is necessary to find the fraction of culture supernatant which is responsible for such effect. However, it is possible that different fractions have synergic effects. Future studies should focus on identification of lactobacilli fractions which confers cytotoxic effects against cancer cells as well as those modulate cancer-related pathways. Furthermore, in this study we just evaluated expression of these targets at mRNA level. As phosphorylation status of different proteins in mTOR pathway is important in regulation of this pathway, future studies should investigate the effect of lactobacilli-derived products on phosphorylation of these proteins.
In addition, we have demonstrated modulation of different parts of Wnt/ β-catenin pathway following lactobacilli treatment in different cell lines. Of note, SFRP2 expression has not been detected in HeLa cells before lactobacilli treatment, but considerably has been upregulated following treatment. As revealed by a former study, restoration of the expression of SFRP2 has resulted in decreased Wnt signaling in CaSki cervical cancer cells, decreased abnormal accumulation of free β-catenin in the nucleus, and inhibited cancer cell growth. In addition, SFRP2 inhibited the expression of three transcription factors involved in the EMT program including TWIST (32). However, in our experiment, upregulation of SFRP2 in HeLa cells has not been accompanied by downregulation of TWIST2 expression. In addition, treatment of HT-29 cells with LRS has increased the expression of SFRP2 while decreased the expression of CCND1. CCND1 has been regarded as an unfavorable prognostic factor for colorectal cancer (33), so its downregulation following LS treatment may be of clinical value. As SFRP genes are regarded as targets of cancer specific hypermethylation in the colon (34, 35), upregulation of SFRP2 expression in HT-29 cells following LRS treatment implies a role for lactobacilli in epigenetic regulation of gene expression, which should be evaluated in future studies.
Additionally, we have demonstrated downregulation of TBLR1, a prognostic marker in cervical cancer with a critical role in the invasion and metastasis (36) in HeLa cells following LRS treatment. However, as such effect has been seen following MRS treatment as well, it is not considered as significant. In addition, CCND1 has been downregulated in HeLa cells after LS treatment. As CCND1 is regarded as a marker of poor prognosis in early stage cervical cancer (37), its downregulation by LS may have a clinical significance.
A previous study has shown that the transformation of HPV expressing human keratinocytes needs activation of the Wnt pathway (38). Furthermore, E6 and E7 have been shown to be involved in β-catenin nuclear accumulation and activation of Wnt signaling in HPV-induced cancers (39). Downregulation of Wnt-β catenin pathway in cervical cancer following lactobacilli treatment in addition to our previous data regarding down regulation of HPV E6 oncogene by lactobacilli in these cells (3) implies that certain lactobacilli strains can defeat cervical cancer by various means.
In addition, we have demonstrated that treatment with LRS can result in downregulation of CCND1 and DVL3 expressions in MDA-MB-231 cells by the factors 22 and 68 respectively. Downregulation of these Wnt agonists by lactobacilli provide a possible explanation for beneficial effects of lactobacilli in the treatment of breast cancer patients. However, DKK3 is regarded as a putative Wnt signaling inhibitor (40) whose expression has not been significantly changed following LS treatments.
5.1. Conclusions
Lactobacilli can modulate expression of mTOR and Wnt/ β-catenin pathways genes in cancer cell lines in a strain specific as well as cell type specific manner. Considering the role of lactobacilli in cancer prevention and treatment, understanding how the lactobacilli-derived products inhibit cancer-related signaling pathways may shed new insights on design and development of novel anti cancer strategies.