1. Introduction and Background
Ageing population is rapidly increasing worldwide. Increasing life expectancy, and rise in geriatric population is associated with a greater number of people being diagnosed with malignancy. It has been observed that more than half of all cancer deaths are in people older than 70 years (1). Analysis of the surveillance, epidemiology, and end results archive by Owonikoko et al. (2) showed a poor overall survival rate in patients with age > 80 years. In a developing nation like India, the scenario is even worse owing to social inequalities, resource constraints and limited access to a well-organized cancer care system for detection and treatment of cancer. According to the national census report of India (2011), 30,831,190 males and 33,998,613 females belong to the age group of ≥65 years that account for 5.5% of the nation’s population. Due to rapid increase in ageing population, approximately 70% of the overall cancer cases in India would be detected in adults with age 65 years or older by 2030 (3). Among all cancers prevalent in Indian male population, lung cancer has the highest incidence and mortality (4). According to Malik et al. (5) about 86% of patients of non-small cell lung cancer (NSCLC) in India present with stage III-IV disease of which around 29% comprises the locally advanced group. The majority of these patients are surgically unresectable. Current treatment guidelines recommend a combined modality approach of concurrent chemotherapy and radiotherapy (CCRT) for these patients (6, 7). Nevertheless, the inextricable physiological and medical challenges, inherent in the geriatric population, often preclude clinicians to plan and deliver such combined modality management in these patients. Ageing is associated with decrease in marrow reserve, drug clearance, and lean body mass. Concomitant comorbidities which compromise functional status, and general health, further add to the challenges (8). Additionally, the newer management strategies of cancer are also not adequately addressed as these patients are often under-represented in clinical trials of newer therapeutic approaches (9, 10). Most clinical cancer trials have had arbitrary upper age limits, thereby resulting in paucity of evidences in the geriatric population (11).
Earlier studies have demonstrated poor compliance rate in elderly population of India with locally advanced head and neck cancers (12) but limited literature exists on geriatric patients with locally advanced NSCLC.
The present retrospective institutional study is aimed at evaluating the compliance, toxicity and survival in the elderly patients (≥ 65 years) with locally advanced NSCLC treated at our center which is a major urban tertiary cancer center in northern part of the country and caters to a population not restricted to its locality but also neighboring states. The age cut-off is guided by the retrospective analysis of elderly subgroup of NCIC BR10 trial (13). To the best of our knowledge, this is the first study evaluating the factors related to poor treatment compliance, toxicities and survival in elderly patients of lung cancer from the subcontinent.
2. Methods
Archive of patients attending lung cancer clinic and radiation oncology department between 2008 and 2013 were collected for demographic and clinical details, imaging findings, histopathology, treatment and outcome in older population (≥ 65 years) with locally advanced NSCLC. All information was inserted in a pre-designed proforma. Staging was done according to AJCC staging system 7th edition based on the available clinical and radiological findings. Patients were stratified according to age (> 70 or ≤ 70), sex (female or male), socio-economic status (lower, middle or upper) based on modified Kuppuswamy’s scale, stage (IIIA or IIIB), performance status (world health organization score 0 - 1 or ≥ 2), baseline hemoglobin level (≥ 11 g/dL or < 11 g/dL), histology (squamous cell cancer, adenocarcinoma or others), serum albumin level (< 3.5 g/dL or ≥ 3.5 g/dL), smoking habit (smokers or non-smokers), treatment interruption (≤ 7 days, > 7days), number of pre-existing co-morbidities (0 - 1 or ≥ 2), and compliance (yes/no) to treatment. Patients were treated as per the institutional protocol with multidisciplinary team approach. The treatment decisions were guided by multiple factors like disease burden, symptoms, performance status, comorbidities and functional reserve of patients. Responses were ascertained clinically and radiologically, and were coded according to revised response evaluation criteria in solid tumors (RECIST) criteria. For treatment outcome, toxicity and survival analysis of patients who had received at least one treatment modality- surgery, radiotherapy (radical or palliative), chemotherapy (at least one course of chemotherapy) or tyrosine kinase inhibitors (TKIs) were included. The neoadjuvant chemotherapy regimens practiced at our center are combination of paclitaxel (200 mg/m2), gemcitabine (1.2 g/m2 day1 and 8) or pemetrexed (500 mg/m2) with platinum agents (commonly carboplatin area under curve 5). For concurrent chemo-radiation (CCRT), weekly low dose cisplatin (30 mg/m2) or carboplatin (AUC2) is the preferable agents. Radiation dose-fractionation schedule of 60 Gy/30fractions/6weeks or 48 Gy/20 fractions/4weeks to the primary tumor was practiced for radical treatment and 20 - 30 Gy / 5 - 10 fractions / 1 - 2 weeks’ time for palliative treatment. Both acute and late morbidities related to treatment were documented. The grading of acute and late toxicity were carried out using common terminology and criteria for adverse events (CTCAE) version 4.0 and radiation therapy and oncology group (RTOG) late morbidity scoring criteria respectively. Compliance to treatment of patients was defined by their ability to complete the intended course of treatment as planned by the multidisciplinary team. Patients with incomplete clinical details and those who did not receive any form of treatment were excluded from the analysis. Descriptive statistics was used for describing demographic and clinical characteristics. Impact of various factors on compliance rate was computed using chi square test (Fisher’s exact test). Survival was estimated by the Kaplan-Meier method and the Cox Regression model (univariate and multivariate) was used to identify significant prognostic factors. Factors which had P value < 0.25 in univariate analysis were subjectto multivariate analysis. Analysis was done using the SPSS ver. 20 (SPSS Inc., Chicago, Illinois). The study was approved by Institute ethical committee (IEC/NP-342).
3. Results
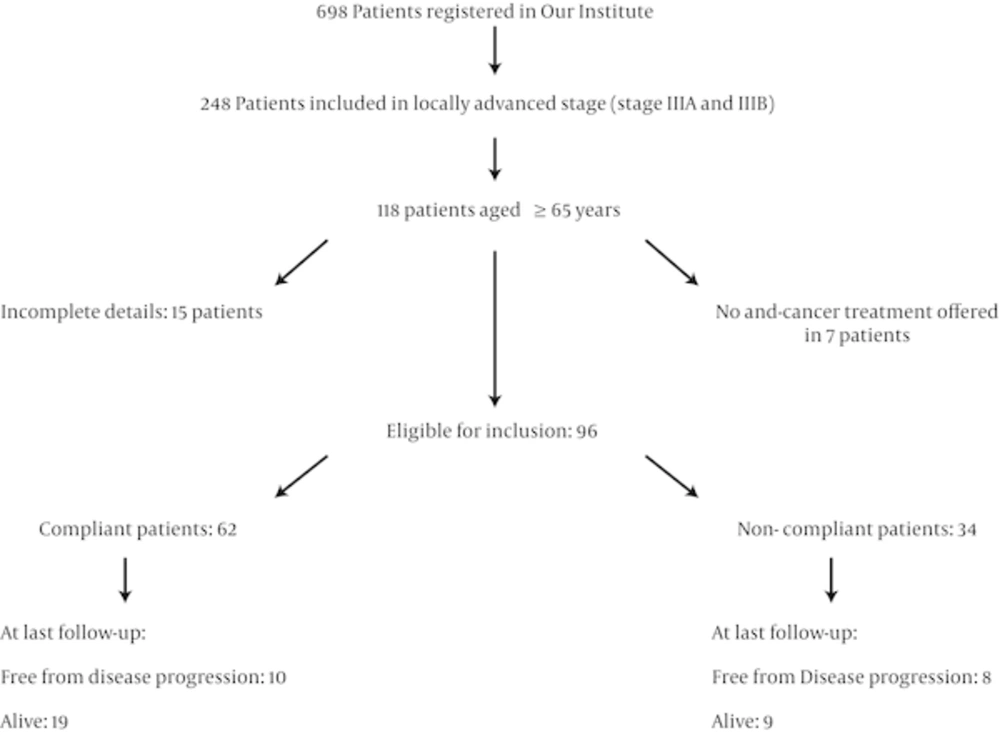
A total of 698 patients of NSCLC were registered for treatment at our institute over the selected study period of which 248 patients were grouped as LA-NSCLC after complete staging work up. 118 patients were in the older population of age ≥ 65 years. Only 96 patients were evaluable as 15 patients were excluded from the current study with insufficient clinical details and 7 patients were not offered any chemotherapy, surgery or radiotherapy and were managed with supportive care to alleviate their symptom (Figure 1).
3.1. Demographics
The median age of this cohort was 72 years (range: 65-82 years). The male: female ratio was 5:1. Around two-thirds of patients belonged to lower and middle socio-economic class. Nearly half of the patients had performance status (PS) score ≥ 2 at presentation. The common symptoms were chest pain (n = 80; 83%) and dyspnea (n = 80; 83%) followed by cough (n = 58; 60%). The median duration of symptoms was 8.24 months. Overall, 57 patients had multiple co-morbidities. The most common co-morbidities were old pulmonary tuberculosis (n = 41), chronic obstructive pulmonary disease (n = 32), systemic hypertension (n = 24) and diabetes mellitus (n = 19). Other chronic diseases include ischemic heart disease (n = 12), rheumatic disorders (n = 8) and reflux disorders (n = 6). The majority of the patients(83%) were smokers. The patient and clinical characteristics are detailed in Table 1.
| Parameters | Group | Number of Patients |
|---|---|---|
| Age | > 70 | 50 (52) |
| ≤ 70 | 46 (48) | |
| Sex | Male | 81 (84) |
| Female | 15 (16) | |
| Socio-economic scale | Upper | 31 (32) |
| Middle | 16 (17) | |
| Lower | 49 (51) | |
| Smoking | Non-smokers | 16 (17) |
| Smokers | 80 (83) | |
| Performance status (world health organization) | 0 - 1 | 47 (49) |
| ≥ 2 | 49 (51) | |
| Stage group | IIIA | 59 (61) |
| IIIB | 37 (39) | |
| Histology | Squamous cell carcinoma | 47 (49) |
| Adenocarcinoma | 37 (39) | |
| Broncho-alveolar carcinoma | 3 (3) | |
| Large-cell carcinoma | 3 (3) | |
| Poorly differentiated carcinoma | 6 (6) | |
| Comorbidities | 0 - 1 | 39 (41) |
| ≥ 2 | 57 (59) |
Baseline Characteristics of the Study Cohort (n = 96)a
Associated pleural effusion was seen in 32 patients and was negative in three consecutive cytological examinations. EGFR (epidermal growth factor receptor) mutation analysis was performed in 23 patients. In PCR (polymerase chain reaction) with direct sequencing was commonly used (n=18) and Amplification Refractory Mutation System (ARMS) PCR technique (n=5) was carried out in a few. Only 3 of them were detected to have EGFR mutations. Baseline hematological investigations revealed grade 1 anemia in 18 patients, grade 2 anemia in 12 patients and grade 3 anemia in 4 patients. Only one patient had baseline pancytopenia. Baseline serum albumin < 3.5g/dL was observed in 35% patients (n = 33).
3.2. Treatment Modality
The commonly practiced sequential chemotherapy regimen consisted of paclitaxel (200 mg/m2) or gemcitabine (1.2 g/m2 day1 and 8) or pemetrexed (500 mg/m2) in combination with carboplatin (AUC 5). For concurrent chemo-radiation (CCRT) weekly low dose cisplatin (30 mg/m2) or carboplatin (AUC2) was preferred. The median number of chemotherapy cycles in the sequential chemo-radiation (SCRT) modality was 4 (range: 1 - 6) and that for CCRT was 4 (range: 3 - 5). The details of intended treatment modality and the number of patients who completed the intended treatment course are shown in Table 2. The median dose of radical radiation was 60 Gray (Gy) in 30 fractions over 6 weeks delivered to the primary disease and involved nodes (range: 45 Gy-60 Gy). The median dose for palliative radiation was 20 Gy in 5 fractions over 5 days (range: 10 Gy-30 Gy). Tyrosine kinase inhibitors (gefitinib or erlotinib) were administered as first line therapy in 4 patients, maintenance therapy in 5 patients and second line salvage therapy in 11 patients.
| Treatment Modality | Number of Patients Planned for Modality | Patients who Completed (% of Planned Patients) |
|---|---|---|
| SCRT | 69 | 46 (67) |
| CCRT | 11 | 7 (64) |
| Palliative RT only | 7 | 5 (71) |
| NACT followed by Surgery | 2 | 1 (50) |
| Surgery followed by adjuvant chemotherapy | 1 | 0 (0) |
| Radical RT | 3 | 2 (67) |
| NACT followed by CCRT | 3 | 1 (33) |
Treatment Details of Study Cohort (n = 96)
3.3. Compliance to Planned Treatment Schedule
The overall rate of compliance to the planned treatment schedule was 65% (n = 62) (Table 2). None of the patients had a progression during first line treatment. The factors resulting in poor adherence to the treatment protocol were stage IIIB (OR: 4.13; 95% C.I: 1.54 - 9.5; p = 0.005), baseline PS score of ≥ 2 (OR: 3.29; 95% C.I: 1.25 - 6.68; P = 0.01) and squamous cell cancer (SCC) histology (OR: 2.09; 95% C.I: 1.07 - 8.09; P = 0.04). Serum albumin < 3.5g/dL (OR: 3.26; 95% C.I: 1.08 - 6.45; P = 0.021) also had detrimental impact on compliance.
3.4. Toxicity Profile of the Study Cohort
Overall rates of hematologic and non-hematologic toxicity were 35% (n = 33) and 25% (n = 24) respectively. The rate of grade ≥ 3 hematological toxicity was 20% (n = 19) and respective rate of non-hematological toxicity was 17% (n = 16). Thrombocytopenia (13.5%; n = 13) was the commonest hematological toxicity followed by anemia (10.4%; n = 10) and neutropenia (n = 7). Five patients developed febrile neutropenia and one patient expired from an episode of pancytopenia with septicemia. Common non-hematologic toxicities were esophagitis (15%; n = 14) followed by skin toxicity (4%; n = 4), radiation pneumonitis (3%; n = 3) and peripheral neuropathy (n = 2). One patient suffered from cisplatin induced grade ≥ 3 nephropathy and was managed with conservative approach. The rate of hospital admission for management of morbidity was 11.5% (n = 11).
The overall incidence of late complications (complications arising after 3 months of completion of radiation therapy) was 12.5% (n = 12). The majority of the late complications (n = 10) were low grade (≤ grade 2 toxicity). The common late toxicities included pneumonitis (n = 4; 4%), brachial plexopathy (n = 3; 3%), esophageal stricture (n = 3; 3%) and skin changes including fibrosis (n = 1), and telangiectasia (n = 1). One patient suffered from grade 4 radiation pneumonitis and another from grade 3 esophageal stricture. Both were managed successfully.
3.5. Survival
At a median follow-up of 18 months (range: 11 - 69 months), the median PFS and OS of the study cohort were 7.4 months and 10.54 months, respectively. The rates of OS and PFS at 1 year were 47% and 27% respectively. The median PFS for compliant and non-compliant patients were 9.72 and 3.3 months respectively. The median OS was 13.72 months and 4.53 months in complaint and non-complaint patients. The median PFS and OS values for patients treated with radical curative intent were 10.21 and 14.3 months respectively. The respective rates of PFS and OS for them at 1 year were 46% and 54%. Univariate analysis demonstrated influence of performance status, stage, treatment interruption, serum albumin and comorbidities on both PFS and OS. On multivariate analysis, serum albumin and presence of two or more comorbidities retained their significant impact on both OS and PFS (Table 3). Socio-economic class had a significant impact on OS on both univariate and multivariate analysis. On multivariate analysis, patients in lower socio-economic strata (hazard ratio i.e. HR: 3.01; 95% C.I: 1.82 - 10.01; P = 0.039), baseline performance status score ≥ 2 (HR: 2.63; 95% C.I: 1.02 - 6.34; P = 0.022), serum albumin < 3.5 g/dL (HR: 3.10; 95% C.I: 1.56 - 6.17; P = 0.008) and presence of two or more comorbidities (HR: 3.81; 95% C.I: 1.37 - 7.10; P = 0.009) were found to have significant impact on OS of patients treated with curative intent. PFS in patients treated with curative intent were influenced by performance status score ≥ 2 (HR: 2.16; 95% C.I: 1.03 - 7.01; P = 0.034), serum albumin < 3.5g/dL (HR: 1.94; 95% C.I: 1.19 - 3.98; P = 0.0001) and presence of two or more comorbidities (HR: 2.39; 95% C.I: 1.27 - 6.79; P = 0.007).
| Parameters | Hazard Ratio for OS (95% C.I) (Univariate Analysis) | HR for OS (95% C.I) (Multivariate Analysis) | Hazard Ratio for PFS (95% C.I) Univariate Analysis | HR for Multivariate Analysis (95% C.I) |
|---|---|---|---|---|
| Age | ||||
| ≤ 70 | 1 | 1 | ||
| > 70 | 1.032 (0.589 - 1.81); P = 0.912 | 1.223 (0.727 - 2.057); P = 0.45 | ||
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 1.36 (0.63 - 2.94); P = 0.432 | 1.27 (0.64 - 2.54); P = 0.49 | ||
| Socio-economic class | ||||
| Upper | 0.93 (0.63 - 1.95); P = 0.19 | 0.68 (0.33 - 2.13); P = 0.89 | 0.47 (0.17 - 12.13); P = 0.91 | |
| Middle | 1 | 1 | 1 | |
| Lower | 3.31 (1.28 - 8.70); P = 0.009 | 2.91 (1.12 - 9.01); P = 0.023 | 1.88 (0.49 - 7.09); P = 0.84 | |
| Smoking habit | ||||
| Non-smoker | 1 | 1 | ||
| Smoker | 1.22 (0.52 - 2.89); P = 0.65 | 1.18 (0.57 - 2.52); P = 0.65 | ||
| Baseline Hemoglobin | ||||
| ≥ 11 | 1 | 1 | 1 | |
| < 11 | 1.58 (0.89 - 2.84); P = 0.629 | 2.81 (1.57 - 5.03); P < 0.0001 | 2.72 (1.40 - 5.29); P = 0.009 | |
| Baseline Performance status (WHO) | ||||
| 0 - 1 | 1 | 1 | 1 | 1 |
| ≥ 2 | 5.40 (2.84 - 8.20); P = 0.001 | 2.33 (0.95 - 5.78); P = 0.051 | 3.74 (2.17 - 6.42); P < 0.0001 | 1.33 (0.58-3.07); P = 0.74 |
| Stage | ||||
| IIIA | 1 | 1 | 1 | 1 |
| IIIB | 2.81 (1.53 - 5.12); P < 0.0001 | 1.015 (0.53 - 2.11); P = 0.69 | 2.15 (1.24 - 3.71); P = 0.006 | 1.12 (0.618 - 2.10); P = 0.87 |
| Interruption | ||||
| ≤ 7 days | 1 | 1 | 1 | 1 |
| > 7 days | 3.75 (2.01 - 6.99); P < 0.0001 | 1.33 (0.59-3.01); P = 0.83 | 3.31 (1.92 - 5.70); P < 0.0001 | 1.34 (0.637 - 2.81); P = 0.86 |
| Serum Albumin | ||||
| ≥ 3.5 | 1 | 1 | 1 | 1 |
| < 3.5 | 2.11 (1.17 - 3.77); P = 0.014 | 2.06 (1.08 - 3.93); P = 0.021 | 1.91 (1.13 - 3.20); P = 0.014 | 2.34 (1.32 - 4.16); P = 0.021 |
| Comorbidities | ||||
| 0 - 1 | 1 | 1 | 1 | 1 |
| ≥ 2 | 3.47 (1.82 - 6.62); P < 0.0001 | 2.54 (1.20 - 5.37); P = 0.007 | 3.89 (2.16 - 6.99); P < 0.0001 | 3.10 (1.56 - 6.17); P = 0.011 |
| Histology | ||||
| Adenocarcinoma | 1.78 (0.47 - 2.49); P = 0.97 | 1 | ||
| Squamous cell cancer | 1 | 1.23 (0.64 - 3.82); P = 0.91 | ||
| Others | 0.89 (0.62 - 3.59); P = 0.82 | 0.87 (0.59 - 2.81); P = 0.67 |
Cox Proportion Hazard Ratio (HR) with 95% Confidence Interval (C.I) for Overall Survival and Progression-Free Survival for Entire Cohort (n = 96)
4. Discussion
The current retrospective analysis was aimed at studying the outcome and compliance to treatment in aged population with LA-NSCLC. The study reveals a treatment adherence rate of 65% among geriatric patients (≥ 65 years) with locally advanced NSCLC. Poor treatment compliance was related to poor PS, low serum albumin level and multiple comorbidities. Low serum albumin indicates poor nutritional reserve and, therefore, influences compliance to treatment. Of note is the impact of histological subtype on treatment compliance. This seeming paradox can be explained by the predominance of smokers among patients with SCC subtype.
The association of multiple comorbidities with poor survival has also been demonstrated by Hsu et al. (14). The authors observed that variables of their comorbidity scoring criteria, age ≥ 80 years, cigarette smoking and PS ≥ 2 were associated with shorter survival duration. Age was also shown to be a poor prognostic factor by Owonikoko et al. (2). The current study could not find such association between age and survival. A borderline impact of performance status score (WHO) ≥ 2 on overall survival of the study cohort was observed with multivariate analysis, although it had a significant impact on survival for the geriatric patients treated with curative intent or radical approach.
The association of low serum albumin with poor OS and PFS is in accordance with Malik et al (5). Low serum albumin indicates depleted nutritional status and may result in poor response to treatment and outcome (15).
Various studies have assessed the influence of prognostic factors like age, stage, PS histology, treatment modality, hemoglobin level and serum lactate dehydrogenase level on treatment but till date none observed the effect of these factors in the elderly population (16). Further studies are required to explore and validate these factors in the elderly subgroup.
In the present study, both PFS and OS are significantly inferior in the non-compliant patients. However, the survival result of compliant patient cohort is fairly good.
The survival results were also superior in patients treated with radical approach. These findings were similar to the results of retrospective analyses of a number of randomized trials that have compared outcomes of chemo-radiation between elderly patients and their younger counterparts (17, 18). Several other studies have also reported efficacy and feasibility of combined modality therapy in elderly population and demonstrated equivalent survival in compliant patients compared to the younger population (7, 19, 20).
Therapeutic options in the geriatric population are often biased as compared to younger population with similar disease burden. This is seen across all tumor types but is of particular importance in lung cancer where the median age at diagnosis is 68 years (21, 22). Also the geriatric patients merit additional careful consideration in view of higher prevalence of co-morbidities and depletion of their physiological reserve which affects therapy adversely. Therefore, as recommended by the Society of Geriatric Oncology, a thorough objective assessment of elderly patients is mandatory (23). This would help to break through the barrier of ageism. Comprehensive geriatric assessment (CGA) is a tool for such multi-dimensional assessment which provides an accurate evaluation of treatment tolerance (24). Use of such tools, thereby, helps the oncologist in optimizing the treatment decision.
The cut-off age of ≥ 65 years in the present study is based upon a subgroup analysis of the NCIC BR 10 trial which included primarily Caucasian patients in North America. This is contrary to the study by Vigg et al. (25) where patients ≥60 years were included for evaluating the clinical spectrum of the lung cancer in the elderly Indian patients. But over the last decade, India has acquired the label of “an ageing nation” with 7.7% of its population being more than 60 years old (26). This is reflected in the finding of median age of 65 years in study of assessment of cancer care in older patients by Sarkar and Shahi (27). Moreover, the median age at presentation in patients with lung cancer is 68 years which further justifies this age cut-off.
The present study shows a negative impact of treatment interruption over survival. However, this association remained unproven on multivariate analysis. Our finding is in agreement with the results obtained by Singh et al. (28) in a cohort of 118 chemotherapy-naïve NSCLC patients. They observed that inter-cycles delay during chemotherapy did not bear any significance on survival. Given the relatively short expected life span for advanced NSCLC (generally 8 - 12 months), short period of treatment interruption does not significantly influence survival.
The correlation of low baseline hemoglobin level with poor PFS can be explained by the relative radio-resistance due to anemia and tumor hypoxia leading to suboptimal disease control (29).
In a developing country like India, quality cancer care is mostly limited to the major cities which compel the patients to travel a long distance for comprehensive cancer management. This results in loss of salary for the care givers and added financial burden to the already existing financial constraints towards cost of treatment. Major centers also face the burden of large number of patients thus creating a substantial waiting time for diagnosis, intervention and therapy (30). Our center is also a well-designed tertiary cancer care center and caters to a large patient population adding to the machine burden and prolonged waiting period for cancer directed therapy. Therefore, factors like socio-economic background, cost of treatment, distance to treatment center and family support also play decisive role for non-compliance and poor survival outcome especially in geriatric population. The present study highlights the influence of socio-economic status on overall survival. Incorporation of a comprehensive assessment tool in the current practice can, therefore, improve the overall treatment adherence, narrow the spectrum of toxicities and improve overall outcome. Small sample size, limited follow-up duration and lack of any comorbidity scoring system are few of the limitations of the present study. The result of the present study therefore needs to be interpreted with caution and obviates the need of further prospective studies in this age group with larger sample size, baseline comprehensive assessment and appropriate scoring of co-morbidities.
4.1. Conclusions
Adherence to treatment is an important factor determining outcome and survival in geriatric population with locally advanced NSCLC. Our study is highlighting that elderly patients (≥ 65 years) with locally advanced NSCLC from a developing nation are susceptible to poor adherence to the planned treatment protocol leading to poor disease control and overall survival. Heavy disease burden, poor performance status, multiple comorbidities, poor socio-economic background and nutritional status are the potential underlying reasons. Use of comprehensive geriatric assessment will enable us to select patients for optimized curative anti-cancer therapy in this age group. A larger prospective trial with comprehensive assessment of clinical, social and treatment related factors which influence treatment adherence as well as survival and toxicities in this age group will be helpful.