1. Background
Colorectal cancer (CRC) has been considered as one of the most common malignancies all over the world and its incidence is increasing slowly (1-3). Dietary, environmental factors and genetic susceptibility play substantial roles in the development of CRC (4, 5).
HER2/neu is located on chromosome 17q21 and encodes a 185 kD transmembrane glycoprotein receptor that is activated by the phosphorylation of tyrosine residues. HER2/neu activation initiates various signal cascades such as the MAPK, PI3K/AKT, and PKC pathways, which are essential for cell differentiation, proliferation and survival during fetal development (6-8). In neoplastic cells, dysregulation of the mentioned pathways and also increased expression of HER2/neu promotes tumor cell growth, progression and migration (9). Overexpression of the HER2/neu receptor is detected in 25% - 35% of breast cancer patients and has been known as an important prognostic and predictive factor (10). In the breast cancer patients, treatment with Herceptin® (trastuzumab), an anti-HER2/neu monoclonal antibody, (as a single agent or in combination with standard chemotherapeutics drugs) proved to reduce tumor volume, to enhance the effects of chemotherapy and to improve the overall survival (OS) rate of the patients significantly (11-13). One of the main mechanisms of function of trastuzumab is that it binds to HER2/neu and causes an increase in p27 level, which has a role in ceasing cell proliferation. Studies showed that trastuzumab is effective only in the manner that the HER2/neu receptor is overexpressed (14).
To extend the potential value of anti-HER2 therapy, the investigators have examined the HER2/neu expression in different non-breast malignancies like ovary, lung, bladder, prostate, pancreas, esophagus, stomach, and colorectal (6). One study exhibited that either HER2/neu protein overexpression or gene amplification is associated with approximately 25% of all cases of gastrointestinal malignancies (15). Bang et al. (2010) expressed that the combination of trastuzumab with conventional chemotherapy considerably improved survival of HER2/neu-positive patients with advanced gastric or gastroesophageal junction cancer (16).
However, incompatible data exist about the prevalence of HER2/neu overexpression in CRC, with a wide range from 0 to 83 % (15, 17, 18). Some authors showed that HER2/neu overexpression had a poor predictive role (19-21). Some reported that HER2/neu was a good predictor marker (22). Others showed that it could negatively relate to survival of patients (17, 23, 24).
Another important issue is that most CRCs with overexpressed HER2/neu level, exhibit cytoplasmic staining and approximately 5% have a cell membrane immunostaining- only this population is responsive to antibody therapy (25).
Although some studies have reported the incidence and clinical consequence of HER2/neu status in the patients with CRC, its clinical importance is not clear exactly. So, the aim of the present study was to evaluate the immunohistochemical expression of HER2/neu and its relationship with clinicopathological parameters like grade and stage and prognostic factors in the patient with CRC.
2. Methods
2.1. Patients and Tissue Specimens
This retrospective study involved 50 patients who underwent colorectal resection at the department of general surgery of Rouhani hospital (Babol, Iran) during the period from 2009 to 2014. The required sample size was estimated by the formula pqz2/d2, with a confidence level of 95% and a degree of precision of 0.05. The study protocol was approved by the hospital’s research ethics committee, and also all patients gave informed consent for storing and upcoming use of their resected tumor specimens for further research purposes. In this study, cases were eligible to be included if they were diagnosed as invasive colorectal carcinoma, according to the WHO classification and experienced colorectal resection to deliver adequate histological sections for proper application of IHC testing (HER2/neu). Excluded were patients with non-epithelial colorectal lesions, those with prior malignancies and those who had received chemotherapy or radiation therapy for CRC pre-operation. For each patient, data about the clinicopathological parameters were collected from the medical records, the pathologists’ reports and the operation data. They included sex, age, clinical history, tumor location, tumor size, gross appearance, presence of distant metastasis, surgical resection margin involvement, tumor grade and stage. Then Paraffin blocks of these cases were collected from the archives of the department of pathology for re-evaluation.
2.2. Tissue Preparation for Histopathologic Examination
Serial sections of 4-micron thickness were prepared from each paraffin block. One of them was stained by Haematoxylin and eosin for histopathological re-evaluation. Tumor grading and staging were done according to the WHO criteria and the seventh edition of the UICC guidelines respectively. Vascular and perineural invasion were also microscopically evaluated.
2.3. Immunohistochemical Staining
IHC staining for HER2/neu was performed with the Dako Hercep test assay (Dako Corp., Carpenteria, CA), according to the manufacturer’s recommendations. Briefly, sections of archived formalin-fixed, paraffin-embedded tissue (4-μm thick) were placed on slides coated with polylysine. Then, antigen retrieval was performed by heating in 10 mmol/L sodium citrate (pH: 6.0) for 15 minutes. The samples were cooled at room temperature for 20 min and washed with phosphate-buffered saline (PBS). Non-specific binding sites were blocked by pre-incubation with 10% fetal calf serum in PBS with 0.01% sodium azide (10 minutes). Endogenous peroxidase was blocked by a 10-min incubation with 0.3% H2O2 in methanol. After that slides were incubated with rabbit anti-human HER2/neu as a primary antibody (Dako Corp, Carpinteria, CA) (dilution 1:600) for 40 minutes at room temperature. Binding was considered by the Dako Quick-Staining, Labelled Streptavidin-Biotin System (Dako), and then by the addition of diaminobenzidine as a chromogen. Counter staining was done with haematoxlin. For negative controls, the tissue sections were incubated with PBS in the absence of primary antibody and for positive controls, invasive breast cancer samples were used.
2.4. Scoring System
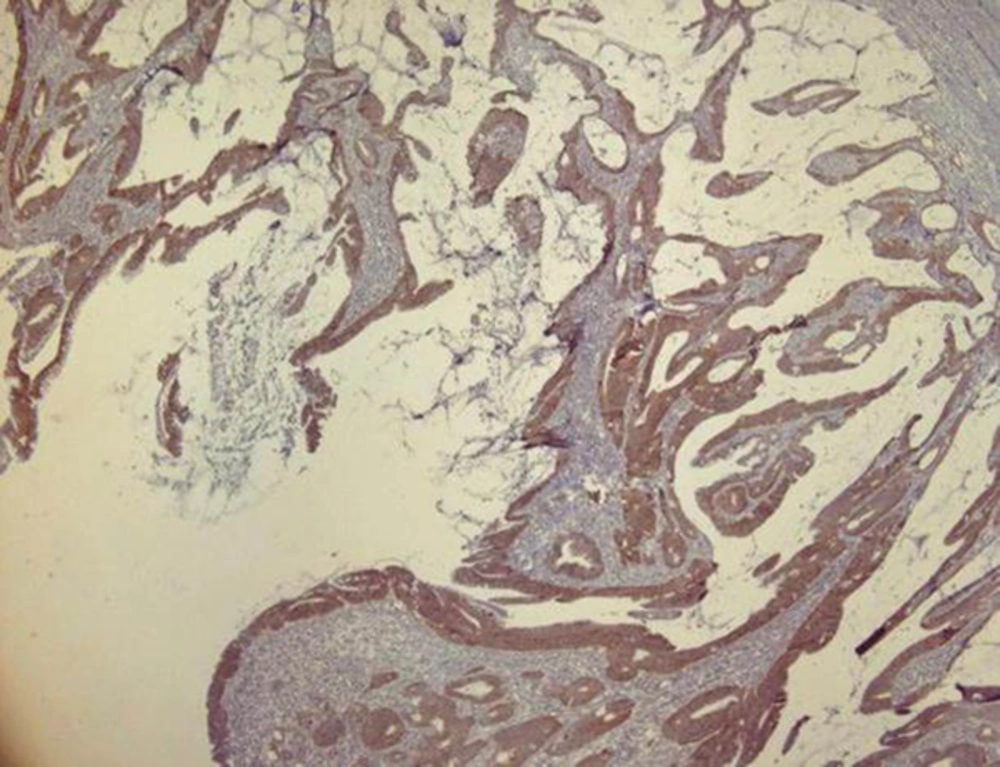
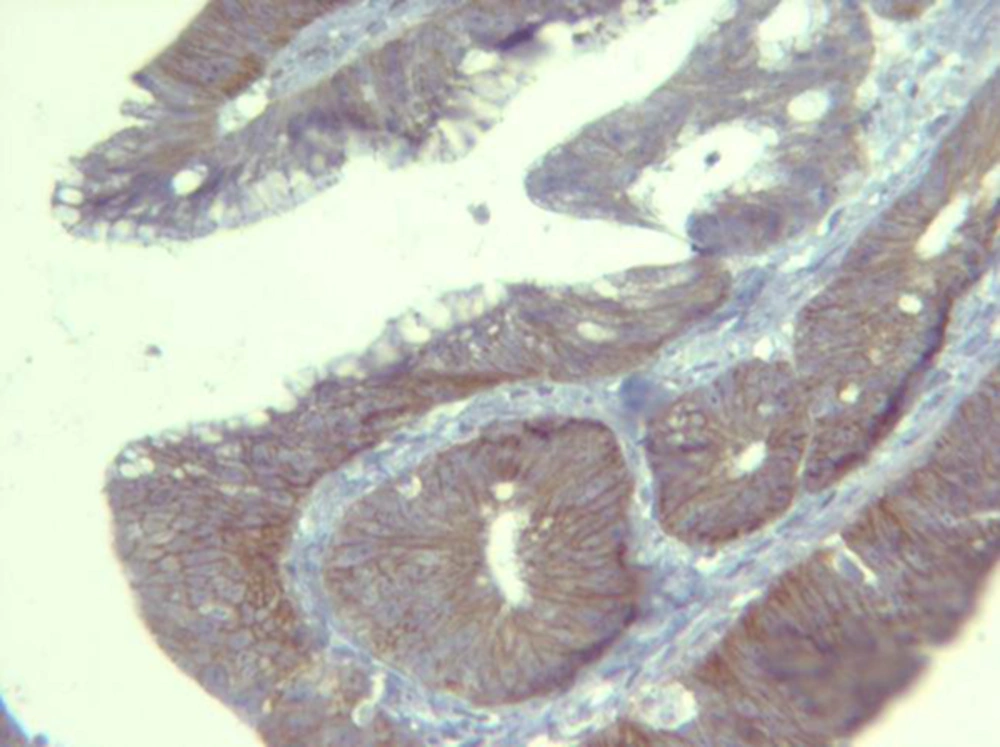
Slides were evaluated independently by two expert pathologist without knowledge of the clinical outcome of the patients. Any disagreements (< 5%) were reviewed, followed by definitive decision. Scoring of the HER2/neu immunostaining was conducted according to published criteria for breast carcinoma, according to the American society of clinical oncology (ASCO)/college of American pathologists (CAP) guidelines. Cytoplasmic or background staining was not graded. The intensity of membranous staining was graded from 0 to 3. Briefly, a score of 0 indicates no staining observed in tumor cells. A score of 1+ indicates weak, incomplete membrane staining in any percentage of tumor cells or weak, complete membrane staining in fewer than 10% of tumor cells. A score of 2+ indicates weak, complete membrane staining in 10% or more of tumor cells or intense, complete membrane staining in fewer than 30% of tumor cells. A score of 3+ indicates uniform, intense, complete membrane staining in more than 30% of tumor cells. The 3+ was chosen as positive expression for HER2/neu. For controlling the heterogeneity, two samples were taken from different areas of the same tumor.
2.5. Statistical Analysis
The data collected were analyzed using SPSS version 20.0 (statistical product for services solutions). Statistical analyses were done by using the Pearson chi-square test, Fisher’s exact test and student’s t-test. P values less than 0.05 were considered statistically significant.
Compliance with ethical standards: the study protocol was approved by the ethics committee at the Babol University of Medical Sciences, Babol, Iran (code: 4447).
3. Results
This study was a retrospective study, conducted on fifty patients with CRC, collected from colorectal resection specimens sent to our pathology department.
Patient included 27 (54%) male and 23 (46%) females with a median age of 60.42 (range: 33 - 85). The lesions were present in the right colon (48%), the sigmoid colon (22%), the left colon (16%), the rectum (8%), and the transverse colon (6%). Most of the studied tumors were 5 cm in diameter or larger (54%) and the ulcerating type was the most common grossly showed feature (64%). All the cases had free surgical margins.
Of all the 50 cases, 26 cases (52%) were well differentiated (grade I) tumor, 15 cases (30%) were moderately differentiated (grade II) tumor and 9 cases (18%) were poorly differentiated (grade III) tumor. In (n = 14) 28% and (n = 11) 22% of cases, vascular and perineural invasion were detected respectively.
The staging of the tumors according to the TNM system revealed that (n = 10) 20%, (n = 24) 48%, and (n = 16) 32% were stage I, II, and III respectively.
Immunohistochemical staining for HER2/neu was performed in all the 50 cases of the CRC, the scoring was done, and the results were taken based on the intensity, pattern and the percentage of HER2/neu expression. Most of them (76%) were HER2/neu negative and in 12 (24%) cases, HER2/neu positive immunostaining were detected. 24% (n = 12), 36% (n = 18), 30% (n = 15), and 10% (n = 5) were scored as 3+, 2+, 1+, and 0 respectively (Figures 1 and 2).
Most of the HER2/neu positive cases (8 cases) were female. There was no significant correlation between sex and HER2/neu positive reaction (P > 0.05).
Most positive HER2/neu patients (58.3%) were older than 55 years and no statistically significant relationship was detected between the scoring of HER2/neu and age of patients (P > 0.05).
Among the positive cases, 66.7% had a tumor with a diameter of 5 cm or larger. No significant relationship was detected between the scoring of HER2/neu expression in tumor cells and tumor size (P > 0.05).
Most cases demonstrated HER-2/neu positivey located in the right colon (58.3%) followed by descending (25%) and sigmoid (16.7%). No significant association was found between the site of the tumor and the expression of Her2/neu (P > 0.05).
Of the total positive HER-2/neu cases, most of them (n = 9) 75 % were Grade I, while only one (8.3%) was Grade II and two cases (16.7%) were Grade III. Analysis of the data showed no significant statistical correlation between the degree of differentiation and the frequency of over expression of HER2/neu marker (P > 0.05).
Among the positive cases, (n = 4) 33.3%, (n = 6) 50%, and (n = 2) 16.7% were stage I, II, and III respectively. However, no significant correlation was found between the stage of tumors and the expression of Her2/neu (P > 0.05).
No statistically significant relationship was detected between the scoring of HER-2/neu expression in tumor cells and vascular and perineural invasion (P > 0.05).
4. Discussion
The molecular mechanisms of CRC depend on both genetic and epigenetic factors (26). Among the different methods for determining the HER2/neu status, the US FDA has accepted only IHC for HER2/neu protein expression and FISH for HER2/neu gene amplification (22, 27).
In previous studies the levels of HER2/neu overexpression in patients with CRC, range from 0 to 83%; however, more recent studies demonstrate that HER2/neu expression is nearly 10%. So it is unclear whether HER2/neu is a potential therapeutic target or/and a prognostic marker in patients with CRC or not (23, 28-30).
In the present study, 54% of the studied cases with CRC, were males and 46% were females, The results are in accordance with some studies (8, 11, 18, 31, 32), but differ from the findings of Cressey et al. (2006) who reported that females had a higher incidence in their study (33).
The ages of our studied patients ranged from 33 to 85 years (mean age 60.42 years), and we showed that 60% of patients were older than 55 years. These results are relatively compatible with the findings of Terzi et al. (2008) (34), Neklason et al. (2008) (35), Office for national statistics (2008) (36), and Kafi et al. (2006) (8).
The most common sites of tumor involvement in the present study were the right colon (48%), the sigmoid colon (22%), and the left colon (16%). This is in agreement with findings of Elwy et al. (2014) (18). However, some researcher reported that the left colon was the most common region for occurrence of CRC (31, 37). Smyrk (2002) indicated a shift toward right-sided cancers occurring during the second half of the twentieth century (38).
According to the histological grade of the CRC, the present study showed that well differentiated carcinoma (Grade I) was more common than other types, representing 52% of cases. This was in accordance with findings of Sharifi et al. (2009) (39) and Kafi et al. (2006) (8). Whereas, some other studies reported that the moderately differentiated carcinoma was the most common type and explained that the variation between different studies may be related to the randomized selection and also small sample size (18, 40). Most cases in our study were stage II, which is relatively in accordance with results obtained by Sis et al. (41) where the highest incidence was in stage B (46.4%).
We estimated the positivity rate of HER2/neu in membranes to be 24%. These results were similar to the findings of some authors (11, 18, 42), who reported that 26%, 27%, and 23% of their patients were HER2/neu positive. However, in a large cohort of CRC, the expression of HER-2/neu was reported in 81.8% patients (17) or Kim et al. (2004) (43) reported HER2/neu overexpression in 0.5% of 185 patients with CRC.
These variations can be attributed to multiple factors like, technical variability in the IHC performance (differences in tissue fixation, processing, epitope retrieval, primary antibody, interpretation and reporting of pathologist, ununified and widely acceptable scoring systems for evaluation of HER2/neu expression), sample size, heterogeneity of study population, racial differences, and varied experimental designs (6, 29).
Another key subject is lack of agreement about whether only membranous or cytoplasmic or both stainings should be considered for evaluation of HER2/neu overexpression. However, the cytoplasmic localization of HER2/neu occurred more frequently, its prognostic value is indefinable (44, 45). Half et al. (2004) studied HER2/neu expression in CRC cell lines and exposed that membrane, but not cytoplasmic localization, was strongly related to HER2/neu gene amplification. They found a significant association between HER2/neu cytoplasmic staining and tumor differentiation (46). In the study of Seo et al. (2011) (29), HER2/neu was positive in 65% of the patients out of which 57.5% were cytoplasmic, with only 7.5% of membranous-cytoplasmic staining and no case showed a pure membranous pattern of staining. In the present study, we used the Hercep test scoring system for breast cancer with strict adherence to the manufacturer’s recommendations for our cases. In the breast cancer these guidelines are very specific as trastuzumab is only capable of binding the extracellular domain of membranous HER2/neu and cytoplasmic HER2/neu would not have any importance for clinical therapy. However, some authors indicated that in CRC cytoplasmic HER2/neu could be related to survival prognosis and cytoplasmic HER2/neu may involve in tumor pathogenesis like membranous HER2/neu in breast cancer, although this has not been confirmed in a large multi-center trial yet (25).
In the present study, no significant correlation was found between sex and age of the patients, and HER2/neu expression. These findings are in agreement with several studies (8, 11, 18, 23, 45), but differ from the others, which showed a significant relationship between the age of the patients and percentage positivity of the HER2/neu staining (31, 47).
In our study, no significant association was detected between HER2/neu expression and tumor location which is in accordance with the reports of Elwy et al. (2012) (18), Pappas et al. (2013) (45), Gill et al. (2011) (31), Kavanagh et al. (2009) (23), and Schuell et al. (2006) (11). However, in some studies, decreasing incidence of HER2/neu positive immunostaining from colon to rectum was detected (11, 48).
No significant association was found between the expression of HER2/neu and vascular or perineural invasion. This is in agreement with other studies (21, 49).
We also showed no relationship between size and overexpression of HER2/neu. However, Demirbas et al. (2006) reported an association between HER2/neu overexpression and tumor size > 5 cm, and vascular lymphatic invasion (50).
No significant correlation between HER2/neu expression and tumor grade was detected. This finding was consistent with several studies (11, 18, 23, 31, 45) although a significant relationship was detected between histological grade and HER2/neu expression in some other studies (8, 17, 49).
We also detected no relationship between HER2/neu expression and the tumor stage which was in accordance with some other researchs (23, 45). Asonuma et al. (2013) (51) examined HER2/neu expression in 121 cases with stage II or III colon cancer by IHC and DISH and no significant correlation between HER2 expression and clinicopathological factors was detected and they concluded that HER2/neu expression might not be a valuable predictive factor in the CRC.
It is important to note that the mentioned studies used different scoring systems and/or different antibodies from what is currently recommended, which makes significant inconsistency in the findings.
In the study of Nathanson et al. (2003), HER-2/neu overexpression occurred in only five cases (3.6%). Neither HER-2/neu overexpression nor gene amplification was correlated with any clinicopathologic features or patients’ survival and they concluded that this oncogene has an uncommon role in the progression and development of this cancer (52). Li et al. (2014) by their meta-analysis, stated that HER2/neu overexpression probably has little impact on CRC survival (6). Recently another meta-analysis revealed that no correlation existed between HER2/neu expression and clinicopathological features including tumor location, grade of differentiation, TNM stages and lymph node metastasis (28). In our study, there was also no significant association between HER2/neu over expression and clinicopathological parameters (age, gender, size, location of tumor, tumor grade and stage). Despite the conflicting results, benefits of neoadjuvant trastuzumab treatment for CRC patients is not clear exactly. However, Sorscher, (2011) (53) showed a marked radiographic response to trastuzumab in a HER2/neu positive CRC case. Ramanathan et al. (2004) (54) in a phase II trial revealed low levels of HER2/neu overexpression (8%) in advanced CRC limits the application of Herceptin as a treatment for advanced stage CRC; however, when the patients with metastatic CRC were treated by trastuzumab and irinotecan, 5 of the 7 cases, who overexpressed HER2/neu, responded to the therapy.
To conclude, the present study showed no correlation between the expression of this oncogene and clinicopathological features. Although our sample size was small, our results were compatible with some large case studies. It is essential to note that most studies used breast cancer criteria for scoring the overexpression of HER2/neu in CRC; however, because of significant differences in biological origins of these cancers, it needs to be exactly clarified by further investigations whether it is suitable for CRC or not. Another limitation of the present study was that we only used IHC, so other techniques, like FISH, are recommended for additional studies. One important issue, in contrast to membranous HER2/neu overexpression is that a significant proportion of CRCs (30% - 50%) show cytoplasmic HER2/neu overexpression in most studies. The identity of cytoplasmic HER2/neu remains uncertain. Some evidence exists that it is derived from the upregulation of promoter-binding proteinsleading to an increase in HER2/neu production. So the prognostic value of cytoplasmic HER2/neu is not exactly clear and further studies with large clinical trials and standardized techniques are desirable to investigate this issue, because if cytoplasmic HER2/neu has a pathophysiological role in CRC, intracellular HER2-targeting compounds, like lapatinib, might be a new treatment choice for the patients having cytoplasmic HER2/neu overexpression.