1. Background
Breast cancer is one of the most prevalent types of cancer across the globe with 1.67 million newly diagnosed cases. It comprises 25% of all cancer cases and is the most prevalent among Iranian women (1). When it comes to incidence, and death rate, populations around the globe vary widely based on age, sex, race, ethnicity, economic-social status, geographic location, and marital status (2, 3).
In Iran, according to the cancer registry system, breast cancer with 24.8% distribution is among the most prevalent cancer types in women. The maximum distribution in Iran is in 4 and 5th life decades, which is at least a decade lower than the global rate (4). As reported by Globocan 2012, there are 9,795 new cases annually (1).
A chief factor in decreasing survival rate in breast cancer patients is recurrence (5). Recurrence risk is measured by a collection of breast cancer natural history, prognostic, anatomic, biological factors, and the type of treatment (6). Seven retrospective studies were conducted on 3585 breast cancer patients to investigate recurrence risk. According to the results, the maximum risk is in the first two years with a steep reduction to 5 years and then it gradually moves on to 12 years (7). Generally, a-third of recurrence cases are loco regional and the rest are distant metastasis (8).
The average rate of survival in patients with breast cancer metastasis is 18 - 24 months and 10% - 15% of patients experience early recurrence which is recurrence within two primary years after the treatment. Also, 50% of patients suffer from late recurrence after 5 years (9). Thanks to advancements in early detection and breast cancer treatments, long time survival is expected and in turn it will be of great interest for researchers (10).
Breast cancer is clinically a heterogeneous disease and is affected by a variety of factors (11). Primary tumor size, lymph node involvement, estrogen, progesterone and HER2 receptors are prognostic markers that play roles in recurrence and metastasis. Yet it seems that their concordance, recurrence likelihood, and metastasis rate vary under the effect of other factors. One can hypothesize that metastasis, especially in more invasive cancer types, is more likely to be an innate feature of the tumor regardless of its direct activity based on its size or local invasion (12, 13). Here, we studied the factors effective on early recurrence less than 1 year of treatment and compared them with prognostic factors effective on late recurrence after 5 years. Moreover, the factors contributing to the patients’ death were studied.
2. Objectives
This study aims to investigate the affecting factors on too early recurrence and late recurrence to identify a subcategory of patients with exposure to higher risk of recurrence, so that more invasive treatments could be prevented and replaced by more appropriate ones. In this way, unnecessary diagnostic tests in care and follow up will be ruled out. Finally, having known effective factors in decreasing survival in patients with recurrence, we must enhance survival and quality of life in patients by adopting effective treatments.
3. Methods
This retrospective study was conducted reviewing data acquired from 1604 female patients suffering from breast cancer diagnosed in cancer research center at Shahid Beheshti University of Medical Sciences. Exclusion criteria were being male, stage 4 of the disease at the time of diagnosis, and pathology of DCIS.
This retrospective study was conducted by investigating medical files of patients by a trained nurse. The data were collected in an excel worksheet. Factors investigated in this study include age, estrogen and progesterone receptor, human epidermal growth factor receptor 2 status, lymph node status, stage of disease, tumor grade, lymph vascular invasion with pathological data registered in patients’ file, survival without disease after primary treatment acquired from the files, recurrence site spotted in examinations, imaging, and biopsy. Statistical data revealed the relation between these factors and breast cancer patients’ survival.
Receptors’ status was determined via patients’ pathological report following primary tumor biopsy. Her2 was determined by means of IHC test. In case the test results were 0 or +1, they were recognized negative. But in case they were +3, they were considered her2 positive. +2 case were re-examined by FISH or CISH tests. Disease free survival (DFS) was defined as the time interval between diagnosis, and recurrence. It was categorized in three groups of 1 year, 1 to 5 years, and more than 5 years.
The patients were treated by surgery (modified radical mastectomy or breast conserving surgery), adjuvant therapy and hormone therapy according to stage of disease and receptor status. Once the treatment course was over, the patients were examined and para-clinically evaluated, every six months for two years and yearly afterwards. In case of clinical suspicion or detection of any symptoms, patients would have undergone tests to identify recurrence, for example, if the patient had some pain in bones or high alkaline phosphatase, bone scan was done. Or in case of suspicion with the liver or abdomen problems, CT scan from abdomen, pelvis or thorax was taken and sometimes biopsy was to identify recurrence. Of these patients, 313 experienced recurrence in the follow up period whose data were collected and analyzed. Recurrence sites were categorized as: 1. Loco regional recurrence including recurrence in breast skin and soft tissues, chest, or in neck lymph nodes, and Axillae; 2. Bone recurrence; 3. Visceral recurrence (involvement of liver, lung, brain and other distant organs). The mentioned variables were analyzed as univariate and multivariate.
This study was aimed to find the factors effective in recurrence in breast cancer patients compared with patients without recurrence, to detect patients with early recurrence (DFS < 1 year) and patients with late recurrence (DFS > 5 years), to compare prognostic factors effective in too early recurrence and late recurrence in these patients, and finally to study factors effective in the patients’ death.
3.1. Data Analysis
The data acquired from the patients was analyzed by STATA version 12. The effects of variables on overall recurrence and then their effects on early and late recurrence was evaluated by univariate and multivariate cox regression model analysis. The data were significant with P < 0.05. Kaplan-Meier curve was used to present the relations between variables with survival without disease (DFS).
4. Results
At the end of the follow-up period, an analysis showed that of 1604 patients with breast cancer, 313 experienced recurrences (Table 1). The median age of patients was 50 years (range: 24.96 - 84.31 years). After breast cancer diagnosis, surgical treatment was done on the patients. 56.79% patients underwent breast conserving surgery and 43.21% underwent modified radical mastectomy. Neo adjuvant chemotherapy was administered to 17.44% patients and 78.10% patients received adjuvant chemotherapy. Radiotherapy was administered to 94.48% patients and hormonal therapy was done in 71.69% total patients and 97.54% receptor positive patients. Among 313 patients with recurrence, 210 patients (67.09%) and 76 (24.28%) were involved with distant and loco regional recurrence. The median follow-up time was 4.33 years (range: 0.005-24.9 years). Table 2 shows distribution of recurrence site. The most prevalent distant metastasis was seen in bone metastasis (40.95% of distant metastasis and 27.4%of recurrence patients). Median interval for recurrence development or DFS was 3.6 years (range: 0 - 24.18 years, standard deviation = 3.77). Disease free survival (DFS) was 96% in one year after treatment and 80% in 5 years. 54% of relapse eventuated between 1 - 5 years after treatment (Table 3).
| Variables of Recurrence | Cases | |
|---|---|---|
| Age | Freq | Percent |
| ≤ 40 year | 103 | 32.91 |
| > 40 year | 206 | 65.81 |
| Unknown | 4 | 1.28 |
| Total | 313 | 100.00 |
| Stage | ||
| 1 | 18 | 5.75 |
| 2 | 99 | 31.63 |
| 3 | 119 | 38.02 |
| Unknown | 77 | 24.60 |
| Total | 313 | 100.00 |
| Lvi | ||
| Negative | 82 | 26.20 |
| Positive | 122 | 38.98 |
| Unknown | 109 | 34.82 |
| Total | 313 | 100.00 |
| ERandPR - a | 93 | 29.71 |
| ER/PR + | 145 | 46.33 |
| Unknown | 75 | 23.96 |
| Total | 313 | 100.00 |
| Tumor size | ||
| ≤ 2 cm | 46 | 14.70 |
| 2 - 5 cm | 108 | 34.50 |
| > 5 cm | 83 | 26.52 |
| Unknown | 76 | 24.28 |
| Total | 313 | 100.00 |
| Nodal ratio | ||
| ≤ 0.25 | 103 | 32.91 |
| > 0.25 | 130 | 41.53 |
| Unknown | 80 | 25.56 |
| Total | 313 | 100.00 |
| Her2 receptorb | ||
| Negative | 150 | 47.92 |
| Positive | 72 | 23.00 |
| Unknown | 91 | 29.07 |
| Total | 313 | 100.00 |
| Lymph node | ||
| Negative | 66 | 21.09 |
| Positive | 247 | 78.91 |
| Unknown | 0 | |
| Total | 313 | 100.00 |
aEstrogen and progesterone receptor.
bhuman epidermal growth factor receptor2.
| Case (%) | Without Other Site | With Other Site | |
|---|---|---|---|
| Bone | 86 (27.4%) | 58 | 28 |
| Lung and pleura | 50 (15.97%) | 38 | 12 |
| Liver | 36 (11.50%) | 28 | 8 |
| Brain | 36 (11.50%) | 27 | 9 |
| Spleen | 1 (0.3%) | 1 | 0 |
| Ovary | 1 (0.3%) | 1 | 0 |
| Total of distant recurrence | 210 (67.09%) | ||
| Local recurrence(soft tissue or chest wall) | 67 (21.40%) | 60 | 7 |
| Regional lymph node | 9 (2.87%) | 8 | 1 |
| Total loco regional recurrence | 76 (24.28%) | ||
| Unknown | 27 (8.62%) | ||
| Total recurrence | 313 (100%) |
| DFS Interval | Total | Recurrence | Lost | Survival | Confidence Interval |
|---|---|---|---|---|---|
| < 1 year | 1604 | 65 | 269 | 0.9558 (95%) | 0.9439 - 0.9651 |
| 1 – 5 year | 1270 | 156 | 674 | 0.7960 (80%) | 0.7702 - 0.8192 |
| > 5 year | 440 | 69 | 371 | 0.5802 (58%) | 0.5316 - 0.6255 |
aDisease Free Survival.
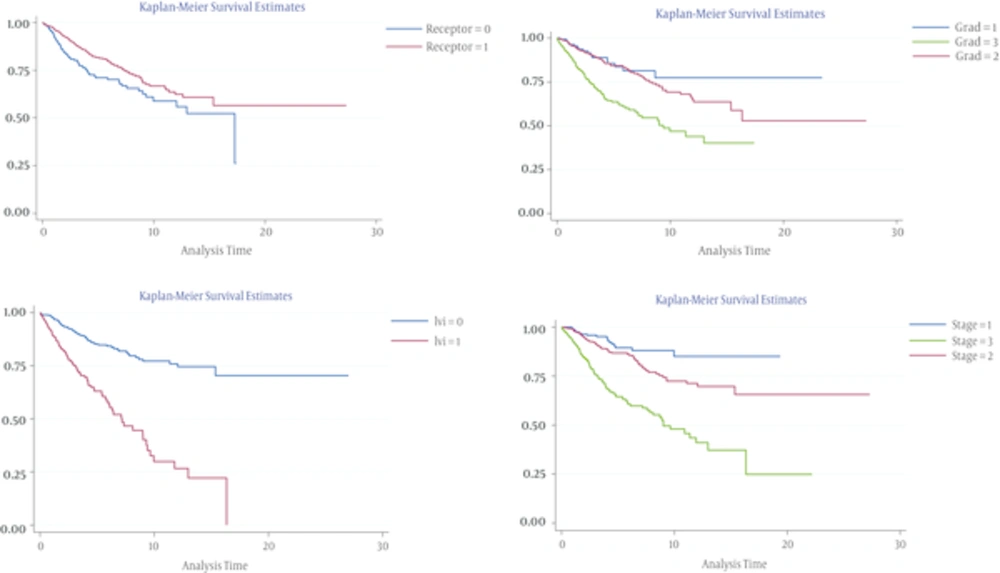
Univariate analysis showed that prognostic factors effective on recurrence including age below 40, higher stages of disease, higher grades, lymph vascular invasion, larger tumor size, negative estrogen and progesterone receptors’ status, and nodal ratio > 0.25 had significant correlations with recurrence (Table 4).
| Variable | Univariate Model | Multivariat Model | ||||
|---|---|---|---|---|---|---|
| -t | Hazard Ratio | P > Z | CI | Hazard Ratio | P > Z | CI |
| Age | ||||||
| ≤ 40 years | 1 | 1 | ||||
| > 40 years | 0.8247517 | 0.509 | 0.4654633-1.461373 | 0.935226 | 0.000 | 0.0348639 - 0.2508747 |
| Stage | ||||||
| 1 | 1 | 1 | ||||
| 2 | 1.93933 | 0.044 | 1.016936-3.698366 | 1.878633 | 0.199 | 0.717592 - 4.918202 |
| 3 | 5.002811 | 0.000 | 2.639037-9.483808 | 3.155098 | 0.054 | 0.9785072 - 10.17329 |
| Grade | ||||||
| 1 | 1 | 1 | ||||
| 2 | 1.395404 | 0.271 | 0.7714729-2.523943 | 0.8933336 | 0.792 | 0.3859236 - 2.067883 |
| 3 | 3.093782 | 0.000 | 1.712921-5.587816 | 1.465748 | 0.388 | 0.6152919 - 3.491703 |
| Lvia | ||||||
| Negative | 1 | 1 | ||||
| Positive | 3.321855 | 0.000 | 2.422798-4.554536 | 1.964151 | 0.029 | 1.047363 - 2.414612 |
| Nodal ratio | ||||||
| ≤ 0.25 | 1 | 1 | ||||
| > 0.25 | 3.302592 | 0.000 | 2.456099-4.440828 | 1.246362 | 0.446 | 0.7074119 - 2.195917 |
| Her2 | ||||||
| Negative | 1 | 1 | ||||
| Positive | 1.133748 | 0.457 | 0.8143957-1.57833 | 0.7898445 | 0.295 | 0.5076873 - 1.228816 |
| Lymph node | ||||||
| Negative | 1 | 1 | ||||
| Positive | 2.792676 | 0.000 | 2.017784-3.865149 | 1.161708 | 0.635 | 0.6250764 - 2.159039 |
| ER/PR positive | 1 | 1 | ||||
| ER and PR negative | 1.4388 | 0.018 | 1.064871-1.944034 | 1.502363 | 0.057 | 0.9882056 - 2.284032 |
alymph vascular invasion.
Stage 3 to 2 (P = 0.000, HR = 5.002)), stage 2 to 1 (P = 0.044, HR = 1.93), grade 3 to 2 (P = 0.000, HR = 3.09), and positive LVI were 3.3 times at higher risk were compared to negative value (P = 0.000). Patients with ER/PR receptors negative were more likely to suffer from recurrence than the ones with positive receptors (P = 0.018, HR = 1.43). Patients with positive lymph node were 2.7 times more than the negative value (P = 0.000) and node ratio > 0.25 were 3 times more than nr ≤ 0.25 in danger of recurrence (P = 0.000, HR = 3.30). DFS graphs of independent prognostic factors that affected the disease free survival time of the patients with recurrence are represented in Figure 1.
In this study, variables effective on early recurrence (less than one year after treatment) and late recurrence (after 5 years later treatment) were studied. 62 (21.60%) and 69 (24.04%) of patients experienced early and late recurrence. 8 variable evaluated with tumor size stratified in multivariate cox regression analysis. The significant prognostic factor was hormone receptor (ER/PR) negative versus positive (Haze .Ratio = 3.54, P = 0.003) and then lymph vascular invasion (Haze .Ratio = 2.65, P = 0.038) (Table 5).
| _t | Haz.ratio | Std.Err. | z | P > z | [95% conf.Interval] |
|---|---|---|---|---|---|
| Age < 40 years | 1 | ||||
| Age > 40 year | 0.6183031 | 0.2645267 | -1.12 | 0.261 | 0.2673219 - 1.430106 |
| Stage | 1 | ||||
| 2 | 0.4089083 | 0.5078259 | -0.72 | 0.471 | 0.35852 - 4.663788 |
| 3 | 1.749409 | 2.481231 | 0.39 | 0.693 | 0.1085453 - 28.19493 |
| Grad | 1 | ||||
| 2 | 1.26732 | 1.345275 | 0.22 | 0.823 | 0.1582434 - 10.14955 |
| 3 | 1.330639 | 1.411012 | 0.27 | 0.788 | 0.1665114 - 10.6335 |
| LVI - | 1 | ||||
| LVI + | 2.651709 | 1.246138 | 2.08 | 0.038 | 1.055636 - 6.660969 |
| Receptor | |||||
| ER and PR + | 1 | ||||
| ER/PR - | 3.547485 | 1.51482 | 2.97 | 0.003 | 1.536197 - 8.19208 |
| Her2 - | 1 | ||||
| Her2 + | 0.8379649 | 0.3816025 | -0.39 | 0.698 | 0.3432381 - 2.045767 |
| Lymph - | 1 | ||||
| Lymph+ | 0.6939046 | 0.6114455 | -0.41 | 0.678 | 0.1233813 - 3.902564 |
We also studied factors effective in late recurrence (after 5 years) and compared them to those of patients with recurrence earlier than 5 years. We found that the most effective factors in late recurrence were stage of disease (stage 2 to stage1, haze ratio = 2.10, P = 0.000 and stage 3 to 2, haze ratio = 8.01, P = 0.000), and lymph vascular invasion (haze ratio = 4.92, P = 0.007) (Table 6).
| t | Haz.Ratio | Std.Err. | Z | P > Z | [95% conf. Interval] |
|---|---|---|---|---|---|
| Age < 40 | 1 | ||||
| Age > 40 | 0.4847364 | 0.263480 | -1.33 | 0.183 | 0.1670446 - 1.406627 |
| Stage | |||||
| 2 | 2.10e+10 | 1.89e+10 | 26.33 | 0.000 | 3.58e+09 - 1.23e+11 |
| 3 | 8.01e+10 | ||||
| Grade | |||||
| 1 | 1 | ||||
| 2 | 0.8064342 | 0.914841 | -1.19 | 0.850 | 0.0872856 - 7.450669 |
| 3 | 0.4746659 | 0.582240 | -0.61 | 0.544 | 0.0428822 - 5.254103 |
| Lvi- | 1 | ||||
| Lvi+ | 4.921768 | 2.90038 | 2.70 | 0007 | 1.550641 - 15.6218 |
| ER/PR+ | 1 | ||||
| ERandPR- | 0.539461 | 0.314967 | -1.06 | 0.290 | 0.1717837 - 1.694098 |
| HER2- | 1 | ||||
| HER2+ | 0.7312887 | 0.438836 | -0.52 | 0.602 | 0.2255769 - 2.370736 |
| Lymph- | 1 | ||||
| Lymph+ | 0.4278776 | 0.304574 | -1.19 | 0.233 | 0.1060257 - 1.726744 |
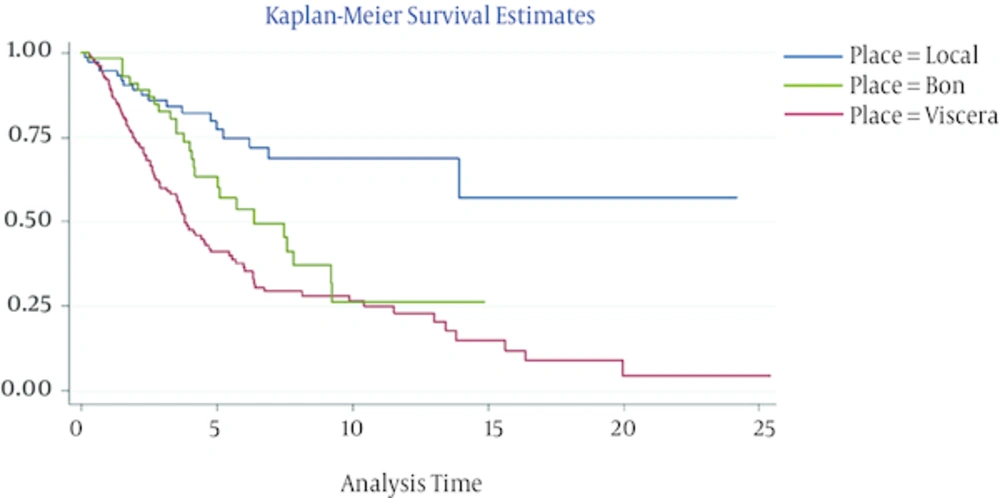
The independent prognostic factors that affected death of the patients after relapse are presented in Table 7. Cox regression analysis showed that the factors affecting the death in patients suffering from recurrence, 10 variables were studied where prognostic factors, such as grade 3 of the disease (P = 0.000, HR = 5.97), and recurrence site and disease free survival. The hazard of death in the patients with visceral recurrence (P = 0.001, HR = 8.04) was more than loco regional and in the patients with bone recurrence (P = 0.031, HR = 4.42) it was more than local recurrence. Patients with DFS more than 5 years (P = 0.001, HR = 0.076) were in the lowest risk as opposed to the ones with DFS lower than 1 year. In Figure 2 represented disease free survival time graph in bases of recurrence site. 56.86% of the patients (178 individuals) survived and 43.13% (135 individuals) died at the end of the follow-up period.
| Variable | Haz .ratio | P > Z | 95% conf. Interval |
|---|---|---|---|
| Age | |||
| ≤ 40 | 1 | ||
| > 40 | 0.1669144 | 0.042 | 0.0296742 - 0.9388759 |
| Stage | |||
| 1 | 1 | ||
| 2 | 4.799454 | 0.404 | 0.1205164 - 191.1338 |
| 3 | 0.7144707 | 0.870 | 0.0125541 - 40.6615 |
| Grade | |||
| 1 | 1 | ||
| 2 | 4.50e+08 | ||
| 3 | 5.97e+08 | 0.000 | 2.52e+08 - 1.42e+09 |
| Lvi | |||
| Negative | 1 | ||
| Positive | 0.4762029 | 0.158 | 0.1699365 - 1.334435 |
| ER/PR + | 1 | ||
| ERandPR - | 1.587114 | 0.334 | 0.6222278 - 4.048245 |
| Her2 receptor | |||
| Negative | 1 | ||
| Positive | 1.413258 | 0.417 | 0.6133221 - 3.256525 |
| Lymph node | |||
| Negative | 1 | ||
| Positive | 3.436266 | 0.069 | 0.9077252 - 13.00826 |
| DFS | |||
| ≤ 1year | 1 | ||
| 2 - 5 year | 0.2367647 | 0.043 | 0.0587704 - 0.9538395 |
| ≥ 5year | 0.0766429 | 0.001 | 0.0157753 - 0.3723626 |
| Site of recurrence | |||
| Locoregional | 1 | ||
| Visceral | 8.040411 | 0.001 | 2.289926 - 28.23158 |
| Bon | 4.424882 | 0.031 | 1.14936 - 17.0352 |
5. Discussion
Breast cancer patients are at recurrence risk even years after receiving treatment which is the highest during the 2 - 3 years after detection of the primary tumor (9). Distant metastasis is the most prevalent form of recurrence and the main cause of death in breast cancer patients. Prognostic factors, estimated to cause recurrence and distant metastasis following the treatment in stages 1, 2, and 3 of breast cancer, include pathologic of breast cancer, tumor grade, tumor size, involvement of lymph node, and hormone receptors’ status of ER, PR, and HER2 (13, 14). In a study conducted on recurrence factors, involvement of lymph nodes, tumor grade, tumor size, and age were strong predictors of recurrence, and estrogen receptors had a medium effect (15).
Our study, a retrospective study, analyzed 313 breast cancer patients from 1604 women suffering from breast cancer in Iran.In our initial investigation of effective factors in recurrence, similar to previous studies, the most effective factors in breast cancer recurrence were lower ages, involvement of axillary lymph node, negative estrogen receptor, higher grades of tumor, higher stages of disease, and lymph vascular invasion.
Akbari et al. in a study on 258 breast cancer patients, concluded that the recurrence risk factors are negative estrogen receptor, and lymph vascular invasion, but other demographic, pathologic and biologic factors (e.g. progesterone, HER2 and P53) were not significantly effective (16).
In recent decades, due to the increase in survival in breast cancer patients as a result of advancements in diagnosis and treatment methods, studies have moved towards investigation of effective factors in early and late recurrence. In a study by Erick Sta et al. which focused on factors effective in less than 2-year recurrence, the major factors effective in early recurrence were tumor stage, and size and lymph node status. This study showed that her2 receptor overexpression was not the only determining factor, but triple negative breast cancer patients were likely to experience early metastasis (9).
Although several studies in Iran have investigated effective factors in recurrence and metastasis (17-19), studies conducted on effective factors in early and late recurrence are scarce. In this study, we examined effective factors in recurrence (as mentioned earlier), and effective prognostic factors in too early recurrence (less than 1 year) and longer than 5-year recurrence.
Tumor size and the number of involved lymph nodes which shows the expansion of the tumor, were pronounced the most effective prognostic factors in breast cancer recurrence (20). However, too early recurrence of tumor as a result of the treatment is considered a factor beyond the recurrence risk factors. Previous studies showed that nodal stage in early recurrence was earlier than 5 years (21). And the most effective factor in late recurrence after 5 years was advanced stage of primary tumor (22).
The most important factors in early recurrence, in a study conducted to examine the effective factors in recurrence earlier than 5 years and later than 5 years on positive receptor patients, were higher nodal stage, higher histologic grade, and age < 35 years, which had insignificant effects on late recurrence. The effective factor in decreasing late recurrence in these patients was endocrine therapy (23). Moreover, another study in this area concluded that lymph node status and tumor size were predictive factors of late recurrence and death in patients with positive receptor after menopause (24).
Multivariate analysis showed that the most effective factors in early recurrence among our patients were estrogen and progesterone receptor status, and the most effective factor in late recurrence in these patients was stage of disease. Lymph vascular invasion was the common effective factor in both groups. In this study, exploring differences between effective factors in early and late recurrence showed that the most effective factors in too early recurrence were the tumor biologic factors (estrogen and progesterone receptor negative), but we did not observe receptor effects in late recurrence. The reason is that the biggest effect of estrogen and progesterone receptor status in increasing recurrence risk is in the first 3 years after the treatment and goes after that (25). We also found that the most effective factor in late recurrence after 5 years was stage of disease which is in line with the findings of some other studies (26).
Many studies on hormone receptors have shown that negative estrogen and progesterone receptors increase response to weakly treatment, among breast cancer patients (27, 28).
Although breast cancer survival has increased together with recent advances in early diagnosis techniques and adjuvant treatments, metastatic patients have varied survival time, because the difference in survival time, determining the prognostic factors for these patients is important.
Bone metastasis is the most common type of distant recurrence in breast cancer patients (29). Our study supports these findings. In the present study, 67.09% (210 patients) were involved with distant organ metastasis and 24.28 %( 76 patients) involved with loco regional recurrence. The most common type of distant metastasis is seen the bone metastasis (40.95% of total distant metastasis).
Patients with bone metastasis generally have longer survival time as compared with the patients with other organ distant metastasis (30). Several studies showed that the patients with longer interval of recurrence development had longer survival (31). Factors affecting the prognosis in the people with recurrence are shorter DFS, recurrence site, the extent of involvement (single or multiple), hormone receptors status, and level of response to treatment (26).
A recent consensus paper reported that multiple factors including HER2 status, site/extent of recurrence or metastasis, DFS, and adjuvant treatments are effective in patients’ prognosis (32, 33).
In search of factors affecting death in patients with recurrence, this study found that factors such as higher grades of tumors, recurrence site, and DFS played a significant role. Patients with loco regional recurrence had a mean DFS of 4.3 years (CI = 3.27 - 5.40) and those with visceral recurrence had a mean DFS of 3.3 (CI = 2.7 - 4.02), which in turn causes better prognosis in patients with longer DFS (with loco regional recurrence). Risk of death in visceral recurrence was more than loco regional recurrence and in patients with bone recurrence, it was more than loco regional recurrence but less than other distant metastasis or visceral metastasis.
Patients with tumor grade 3 had a higher risk of death than those with grade 1. In terms of DFS, patients with DFS > 5 years had the best prognosis and lower death risk compared to patients with DFS < 1.
A drawback of this study was that we did not investigate adjuvant therapy in recurrence risk which should be considered to avoid bias in future studies, it. Future research can be conducted to study more patients with recurrence as well as treatment types. According to the findings of this study, a better understanding of invasive tumor features in breast cancer patients together with the risk factors in individual patients can lead to individual treatments not general guidelines. Because each patient has a unique set of demographic, clinic-pathologic and biologic factors which create a whole different context for recurrence.
5.1. Conclusion
This study was aimed to find risk factors of early and late recurrence in breast cancer patients; so that it can detect subcategories of patients with recurrence risk to better choose the treatments and provide better follow up and care to minimize the risk of recurrence and increase survival. Also, to avoid unnecessary treatments, tests and imaging in follow up in the case of lower risk patients with the purpose of minimizing expenses and physical side effect.