1. Background
The majority of the older population live in developing countries where the number of older people is projected to grow by 250 and 71% in developing and developed countries, respectively within the next 30 years globally (1). The proportion of people aged over 65 is growing faster than any other age groups because of both longer life expectancy and the decline of fertility rates. As predicted by WHO, Chile, China and the Islamic Republic of Iran will have a greater proportion of older people than the United States in 2050 (2). Non-communicable diseases are responsible for more than 87% of the burden of diseases and 60% of all cancers diagnosed in the elderly. This could be explained through the dose-duration effects of carcinogenic exposures and the vulnerability of the elderly to cancer (3). The total cancer incidence rate for the elderly men is four times the rate of 45-64 year old men and twice that of women (4). The incidence of cancer in the 65- and- older population is 10 times higher compared to the younger population (5). The incidence of cancer is estimated to increase in the next decades particularly in the less developed countries due to global aging. Over 50% of cancers occur at the age of 70 and above; however, this gradient reduces in the oldest age group (6, 7). A relationship was found between aging and cancer even with minimum environmental carcinogens; and as a consequence, more human and monetary resources are required to investigate the cancer etiology, prevention, control and quality of life in the elderly (8).
Geographic Information System (GIS) and spatial analysis have provided new opportunities for the policymakers to investigate the relationship between health and environmental risk factors or the geographic characteristics (9-14). Spatial analysis allows us to identify the patterns, and classify data as regular, random, or clustered (15). Spatial autocorrelation is used to assess the rates, and Moran’s I is one of the best statistical measures that is similar to the Pearson’s correlation coefficient. The I statistic measures the covariation between the rates of adjacent regions (16, 17). The current study aimed to examine cancer incidence rates in those over 65 years of age living in Tehran, conduct cluster and Outlier analysis, to learn about disparities across the 22 districts of Tehran and to find common cancer types among the elderly in this mega city.
2. Methods
Tehran metropolitan area is situated on the southern slopes of the Alborz Mountains at a latitude of 35°45′N and a longitude of 51°25′E. Tehran consists of 22 municipal districts and a population of 7,803,883 (according to 2006 census) with 5.9% over 65 years of age.
In the first step, we gathered data on 7,948 new cancer cases and 3,104 elderly cases in 2007 who were over 65 years of age and were diagnosed with cancer from the national cancer registry (NCR), which is published by the Ministry of Health in Tehran. It is noteworthy to mention that in Iran the ministry of health collects cancer data from all public and private hospitals and pathology centers nationwide continuously. We gathered data on the elderly population of the districts from the national statistical center database. The data on cancer cases had to include the patient’s age, sex, address, and cancer type, with topology.
Home addresses were obtained from NCR and mapped at districts level. We excluded the cases with no home address or phone number from the district maps, but we counted them in the calculation of the overall incidence rate.
Age standardized rate (ASR) per 100,000 people was calculated, using the direct method of standardization to the 2000 WHO world standards (18). We only selected those cancer types that constituted at least 1% of all cancers in the elderly. Various cancer ASRs were identified across the districts. Those districts with high incidence rate are presented in the results section.
Spatial statistics: cluster and outlier analysis (Anselin Local Moran’s I)
Spatial autocorrelation was used to detect any local clustering of cancer among the elderly inhabitants of Tehran. Statistically significant spatial clusters or hot spots, cool spots and spatial outliers were identified, using Anselin Local Moran's I. This tool creates Local Moran's I index, z-score, p-value, and cluster/outlier type (COtype). Moran's I ranged from −1 (indicating perfect dispersion) to +1 (perfect correlation). High positive I index implies spatial clusters, meaning that the locations under the study had similarly high or low values in the neighborhoods. Spatial clusters included high-high clusters (HH COtype is a district with high incidence rate), which are surrounded by districts with high incidence rate, and low–low clusters (LL COtype is a district with low incidence rate), which are surrounded by districts with low incidence rate. In contrast, high negative I index indicated that the location under the study was a spatial outlier whose value was obviously different from the values of their surrounding locations. Spatial outliers included high-low (HL COtype is a district with high incidence rate), which are surrounded by districts with normal or low rate, and low–high (LH COtype is a low value in a high value neighborhood) outliers (19). HH COtype may be considered as a hot spot while LL COtype may be identified as a cool spot, and HL COtype could be regarded as an isolated individual hot spot. The significance of Local Moran’s I was evaluated by z-scores and p-values under the null hypothesis of random distribution for a 95% confidence level. A high positive z-score for a district indicated that the surrounding districts had similar values, and a low negative z-score for a district indicated a statistically significant spatial data outlier (20).
We used inverse distance to measure the spatial relationships among the districts and Euclidean distance method (The straight-line distance between two points), but not the standardization of spatial weights.
Statistical Package: ASR was calculated using STATA software version 12, and we used the WHO 2000 standard population as the standard population. In addition, we used Arcgis 10.3 mapping cluster in the spatial statistics tools to measure the Local Moran’s I. This project was approved by the ethics committee of Iran University of Medical Science (IUMS).
3. Results
The age range was 65 - 100 in men and women. Overall, 3,104 new cancer cases were identified in the elderly in Tehran, 2028 men (65.3%) and 1,076 women (34.7%) in 2007, with overall cancer incidence rates of 862.4 and 474.8 per 100,000, respectively. Two hundred and five cases had neither recorded nor precise address so we could not identify their residential districts; however, they were redistributed to Tehran districts to be counted in the overall incidence rate. One hundred fifteen (5.7% with the mean age of 73.3) men and 69(6.4% with the mean age of 74) women elderly did not register their address. The mean age of common cancers in the elderly men and women was 74.23 (SD: 6.3) and 73.59 (SD: 6.2), respectively.
3.1. The Most Common Cancers
Tables 1 and 2 demonstrate cancer types in a descending order of ASR, constituting at least 1% of all cancers (94.2% and 95% of all cancers) in the elderly men and women. Ten common malignancies among men were skin, prostate, bladder, stomach, colorectal, lung, bone marrow, esophagus, larynx and oral cavity, and they were breast, skin, colorectal, stomach, esophagus, bladder, ovary, uterus, eye and lung in women. Prostate, stomach and bladder cancers were the three most common cancers (42% of all cancers) in the elderly men except for skin cancer. Breast, colorectal and stomach cancers were the three most common cancers among the elderly women, accounting for 43% of all cancers. ASR of cancers (per 100,000) in the old age group (65 years and older) and the 30 - 64- year age group is demonstrated in Tables 1 and 2. Based on the total ASR, cancer was almost 6.7 and 2.9-fold more frequent among the elderly than the younger men and women, respectively. Prostate cancer in the elderly men was 14 fold higher than in the younger men because it is increased when a man reaches 50 years of age. Esophagus cancer in the elderly men was around 10.8-fold the corresponding rate in the younger men and 12-fold in the elderly women, but breast cancer was common in both groups (1.5). Bladder cancer was more frequent in the elderly men than in women, and stomach cancer was seven and eight fold more frequent among the elderly men compared to younger men and women. Colorectal cancer incidence was similar in men and women, particularly among younger ages (14) than among the elderly (86 in men and 60 in women).
| Cancer Types | ASR in the Elderly | ASR in the Younger | Districts with a High Incidence Ratea | Districts with Significance Z-Scores and Their Cluster/Outlier Type (COType) |
|---|---|---|---|---|
| Skin | 178.6 | 29.64 | 16,20,3,19,15,7,4,5 | 20(HH), 17(LH) |
| Prostate | 166.25 | 11.55 | 6,1,22,3,2,5,7 | 6(HH), 3(HH), 2(HH), 1(HH), 16(LL) |
| Bladder | 98.63 | 9.32 | 6,2,5,1,3,11,12,22,21 | 6(HH), 2(HH), 18(LL), 9(LL) |
| Stomach | 97.77 | 12.07 | 20,16,4,14,21 | - |
| Colorectal | 86.13 | 14.04 | 2,6,5,21,12 | 2(HH), 5(HH), 21(LH), 22(HL) |
| Lung | 39.87 | 5.38 | 12,21,13,5,6,7,1,20 | - |
| Bone marrow | 28.44 | 5.36 | 22,14,3,6,10,5,1 | 22(HL), 21(LH) |
| Esophagus | 23.4 | 2.15 | 20,16,18,9,19 | - |
| Larynx | 19.21 | 2.82 | 11,20,3,14,1 | 12(HL), 17(LL) |
| Oral cavity | 13.66 | 2.27 | 3,13,19,8,6,15 | - |
| Soft tissue | 10.56 | 1.63 | 20,12,11,14,18 | 20(HL) |
| Brain | 9.39 | 4.28 | 22,3,18,9 | 22(HL), 21(LH) |
| Lymph node | 8.83 | 4.53 | 6,13,14 | 6(HH), 7(HH), 13(HH) |
| Eye | 8.65 | 2.84 | 13,16,19,10 | - |
| Total | 862.4b | 128 | 6,3,5,1,2,20,12 | 2(HH), 9(LL) |
aThe highest rate is the first district in tables.
bAll Cancers in the Elderly Men.
| Cancer Types | ASR in the Elderly | ASR in the Younger | Districts with a High Incidence Ratea | Districts with Significance Z-Scores and their Cluster/Outlier Type (COtype) |
|---|---|---|---|---|
| Breast | 107.78 | 70.31 | 1,2,3,6,4,18 | 3(HH), 1(HH) |
| Skin | 81.86 | 15.59 | 22,17,5,15,11,14 | - |
| Colorectal | 60.66 | 14.05 | 11,1,5,3,2 | 11(HL) |
| Stomach | 40.45 | 5.66 | 22,18,15,5 | - |
| Esophagus | 19.87 | 1.64 | 22,15,19,18 | - |
| Bladder | 19.47 | 2.61 | 20,5,1,3,4 | 20(HL) |
| Ovary | 16.88 | 6.5 | 4,1,11,2 | - |
| Uterus | 15.69 | 6.82 | 1,6,9,16 | 9(HL) |
| Eye | 14.3 | 2.18 | 22,16,13 | 22(HL), 21(LH) |
| Lung | 11.42 | 3.12 | 22,1,15,19 | 22(HL), 21(LH) |
| Cervix | 10.61 | 5.05 | 19,18,16 | - |
| Oral Cavity | 10.16 | 1.18 | 21,11,12,19,18 | 21(HL), 22 (LH) |
| Bone Marrow | 10.06 | 2.96 | 22,2,4,9,3 | 22(HL), 21(LH) |
| Liver | 6.67 | 0.98 | 18,1,12,5,3,20,4,2 | - |
| Lymph Node | 6.14 | 1.98 | 2,1,5,12,20,8,14,6,7 | 2(HH), 5(HH) |
| Thyroid | 6.04 | 5.05 | 16,6,1,20,4,3,15,5,14,2 | 20(HH) |
| Total | 474.8b | 164.4 | 22, 1,2,6,11,5,4 | 22(HL), 21(LH) |
aThe highest rate is the first district in tables.
bAll Cancers in the Elderly Men.
3.2. Variation Among Districts
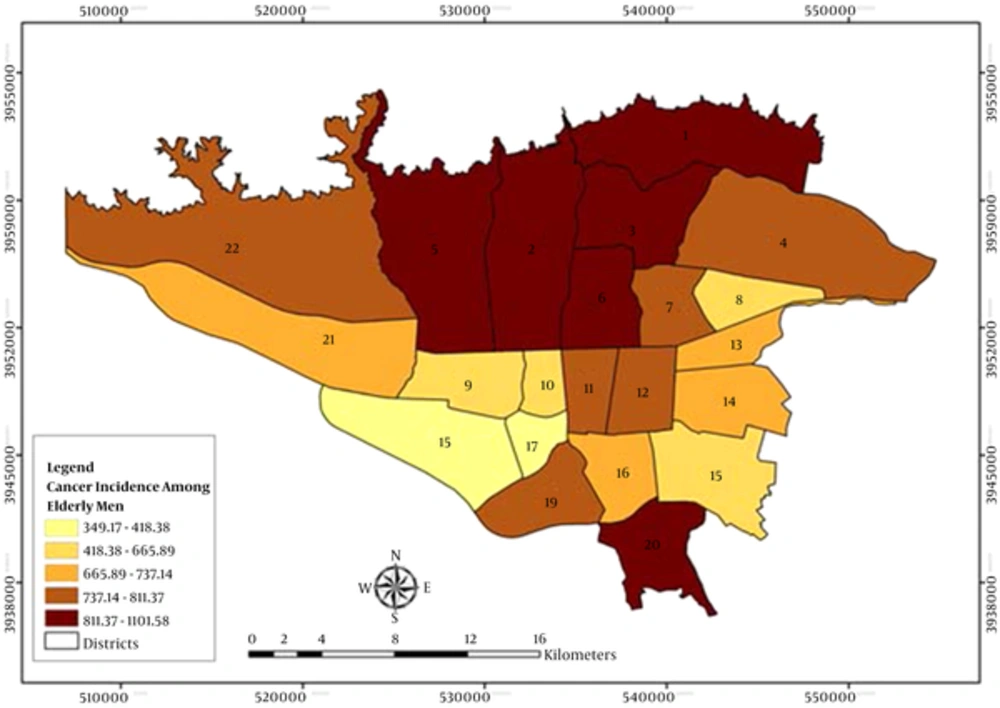
The highest rate of bladder and prostate cancers in men were found in district 6 (center), stomach and esophagus cancers in district 20 (south), skin in district 16 (south), oral cavity in district 3 (north), lung in district 12 (center), larynx in district 11 (center), colorectal in district 2 (north) and bone marrow in district 22 (west) (Table 1).
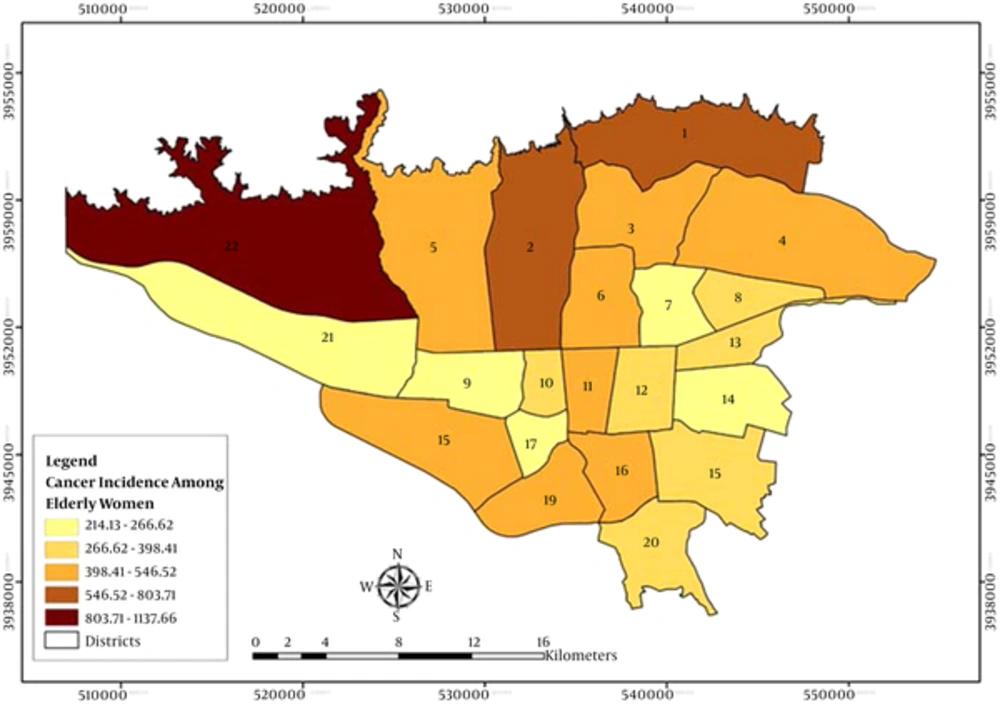
In females, higher breast and uterus cancer incidence were observed in district 1(north), stomach, lung, skin, esophagus and eye cancers in district 22 (west), bladder in district 20 (south), colorectal in district 11(center) and ovarian cancer in district 4 (east) (Table 2).
3.3. Spatial Analysis
Figures 1 and 2 demonstrate the geographic distribution of the age standardized incidence ratios (ASRs) in the 22 districts of Tehran among the elderly men and women, respectively. Using the Moran’s I, we identified more spatial clusters in men compared to women. Hot spots were located in the north and cool spots in the south and west south of Tehran. The most common cancers in men included skin, prostate, bladder, colorectal and lymph node cancer, and in women, they were breast, thyroid and lymph node cancers. Cool spots existed only in male cancer patients with prostate, bladder and larynx cancers. The results revealed two clusters for the total cancer between men: hot spot in district 2 with ASR of 967 (P = 0.015) and another cool spot in district 9 with ASR of 654 (P = 0.042). Moreover, we found an isolated hot spot for the total cancer in district 22 with ASR of 1,137 in women (P = 0.001). Furthermore, districts 2 and 6 were hot spots and districts 9, 16 and 17 were the cool spots for men, but there were lower hot spot clusters for women. They did not have any cool spots while there were more outliers (Tables 1 and 2).
4. Discussion
This study has provided information on the incidence, distribution and spatial autocorrelation cancer in the 22 districts of Tehran. Spatial clusters existed among some districts in some cancer types, indicating that more research needs to be conducted on cancers among the elderly people.
In many of the experimental studies, sensitivity to carcinogens was different among cancers when aging was considered. For example, sensitivity increased in rodent cervix, vagina and subcutaneous tissue, but it decreased in mammary gland, colon, thyroid and ovarian, and it remained stable in lung (21-23). All cancers were higher in the elderly men compared to the 30 - 64- year old age group whilst this rate was lower in women. The proportion of all cancer cases was 110% among the elderly men, and it was 45% in women. Moreover, ASR cancer was almost 6.7 and 2.9-fold more frequent among the elderly men compared to younger men and women, respectively. Based on the data obtained from cancer registries in 51 countries, the proportion of all cancers among the elderly was 61 and 56% in men and women, respectively. Also, standardized rates were approximately seven and four times more in men and women, respectively compared to the middle aged (30 - 64- year old) group (24). The gender proportion incidence rate was 1.81 in our study, which was exactly the same as those of 51 countries in a previously published report (24). In addition, a nearly double incidence rate sex ratio (men to women) was found for the elderly (1.82), but it was almost neutral among the younger individuals (0.78). This may show the impact of highly unusual occurrence of prostate cancer among younger men (25).
The incidence of cancer in ageing populations differs by cancer types. And there are different explanations for this trend. Hansen (1998) reported that prostate, lung and colon cancers were the most common cancers in the elderly men, while the most common cancers in the elderly women were breast, colon, lung and stomach cancers. However, based on our findings the common cancers in men were prostate, bladder, stomach and colorectal cancers; and in women, they were breast, colorectal and stomach cancers (skin cancer excluded) in Tehran. Women were less likely to suffer from colorectal cancer, where the gender ratio was 1.32 in Tehran elderly residents compared to 1.4 in 51 countries (24, 26). Prostate cancer in the elderly men was approximately 22 times more than in the younger men because its incidence increases after a man reaches 50 years of age; and in our study, it was 14 fold higher in the elderly compared to the younger men.
The incidence rate of prostate cancer varies because of the widespread prostate specific antigen (PSA) testing in some developed countries (27-29). In some studies, no evidence of an increase in the incidence along with an increase in the rate of PSA testing (30) was found, but it was higher in the urban and more prosperous areas (31-33); however, this result was not compatible for all areas (34). Also, it may be proposed that prostate-specific antigen testing (PSA) is responsible for an increase in low-grade prostate cancer in advantaged men, but it does not explain the overall increases in all grades as 37% lower incidence was revealed among the most disadvantaged compared to the most advantaged (35). Although higher levels of SES were significantly associated with an increased risk of prostate cancer (36, 37), no evidence was found on the racially uniform population of Caucasians who received free health care (38).
In this study, prostate and breast cancers beside lymph node cancer had the most hot spot clusters. Hot spots were located in the north of Tehran where districts are more privileged, and more cool spots were located in the south of the city where districts are more underprivileged (39). Prostate cancer clustered in districts 1, 2, 3 and 6, and breast cancer in districts 1and 3, in the more privileged areas.
Breast cancer is the most common malignancy in Iran among elderly women, while its ASR is lower than developed countries (ASR = 18.4 v 39, according to GLOBOCAN 2008) (40). Various risk factors either environmental, genetic or life style ones have been attributed. In a study conducted in Massachusetts, an association was reported between drinking water contaminated by wastewater effluent and breast cancer. This study suggested an increased association between broad latency time and more exposure length (41). In addition, a study in Midland found that higher breast cancer rate is spatially associated with soil dioxin contamination (42). Results from a case-control study in Tehran (Iran) revealed that obese women had a threefold increased risk of breast cancer controlling for age, age at menopause, family history of breast cancer and parity (43). In a study, 20 to 30% higher breast cancer incidence was related to women with the highest SEP (44). However, in some studies this increased incidence was mediated partly by reproductive factors, hormone replacement therapy, alcohol and SES (45, 46).
Using the Moran’s I, we identified the hot spot of lymph node in districts 6, 7 and 13 in men and 2 and 5 in women. There was a significant clustering of Hodgkin lymphoma in four of the five study counties, but the dispersed clusters suggest a late exposure to universal environmental agent (47). Non-Hodgkin's lymphoma (NHL) has a significant positive association with solar radiation, but previous ecological results revealed that it may reduce the risk of cancer (48).
Bladder cancer had two hot spots (districts 2 and 6) and two cool spots (districts 9 and 18) for men. Also, there was an isolated hot spot (district 20) for women. Del PilarDiaz reported a spatial distribution of bladder cancer in the urban areas for men, and in the rural ones for women (49). Vieira et al. found a significant bladder cancer “hot spot” in the southwest of the Massachusetts military reservation. They reported an overlap between the bladder cancer hot spots and ground water plumes (50). Environmental factors such as exposure to the chemical components of industrial processes and natural arsenic contamination have been linked to the risk of bladder cancer (51, 52). These risk factors may be justified in unusual area-specific patterns.
ASR of colorectal cancer was found to be higher in men compared to women, and this may be due to the more prevalence of inflammatory bowel disease in men (53). There were spatial hot spots for colorectal cancer only between men in districts 2 and 5. Some studies found that the high level of socioeconomic status was associated with the development of colorectal cancer (54, 55). In Malaysia, colorectal cancer (CRC) hot spots were located in the northwest region of Kuala Lumpur (KL) where residents have high socioeconomic status (56). In another study in Massachusetts men residents had more clusters than women, and after adjustment for SES and urban proportion, some of these areas were not significantly different (57).
There was a spatial hot spot for thyroid cancer in district 20 in women. Radiation exposure in medical procedures, atmosphere, ground water and energy plants (58-62) and cancer chemotherapy (63) may play a role in thyroid cancer. Also, exposure to chemicals used in the industry process and exposure to pesticides (64) are known as environmental risk factors in pathogenesis.
However, to prevent any misconception from our findings presented here, it is noteworthy to mention that we used grouped data. Therefore, the results should be interpreted with caution to avoid ecological fallacies. Further studies may shed light on the proper associations of environmental, socio-economic and other related risk factors with common cancers in Tehran.
4.1. Conclusions
The most common cancers in women remain steady with an increase in age. More hot spots were located in the north of Tehran where the districts are more privileged and more cool spots were located in the south where the districts are more underprivileged. Due to the multi-cause and unknown latency period for different cancers, it was not possible to confirm the significant exposures in particular locations or situations. However, the investigation of cluster reports is useful to detect risky region and to focus on the services for future prevention.