1. Introduction
Worldwide, approximately 80,000 new cases of nasopharyngeal carcinoma (NPC) are diagnosed annually (1). The geographical distribution of NPC incidence is rather distinct and well-defined all over the world (2). Although it rarely occurs in most areas of the world, NPC is among the most common cancers in Southeast Asia and China (3, 4). Currently, WHO classifies nasopharyngeal carcinoma into keratinizing squamous cell carcinoma, non-keratinizing carcinoma, including differentiated and undifferentiated variants, and basaloid SCC (5). NPC is also a distinct entity among head and neck cancers showing strong relation with Epstein Barr virus (EBV) and remarkable sensitivity to radiotherapy and chemotherapy (6). Several prognostic factors have been defined for nasopharyngeal carcinoma that can influence response to therapy and eventual outcome. These factors may be categorized as follows: (a) prognostic factors related to the primary tumor like TNM stage, gross volume of the primary tumor, (b) prognostic factors related to the patient like age, sex, pretreatment plasma EBV DNA level, general medical condition, and (c) prognostic factors related to the treatment (7, 8). Close proximity to critical structures such as brain stem and optic chiasma makes NPC ineligible for adequate surgical resection (4). The current standard treatment approach for nasopharyngeal carcinoma, according to international guidelines, is radiotherapy alone or with concurrent chemotherapy (9).
The present study was conducted considering the influence of genetic and geographic factors on epidemiology of nasopharyngeal cancer and lack of reliable data on its prognosis and treatment outcome in Iran.
2. Methods
We performed a retrospective analysis of 88 patients with tissue diagnosis of nasopharyngeal carcinoma admitted between 2008 and 2014 to our clinical oncology center (Jorjani cancer center, Imam Hossein hospital, Tehran, Iran). All the medical records of the included patients were investigated and with a previously prepared fact sheet, the following data were collected: age, sex, weight, height, date of pathologic diagnosis of the disease, primary tumor site, clinico-pathological characteristics of the primary tumor, date of last follow up, and death (if any).
For the patients, who had not undergone regular follow-ups after treatment, phone calls were made to determine their survival status. For those who were not reachable by phone, the last recorded visit was considered as their last follow-up.
The characteristics of the study population were then described and the overall survival (OS) of the patients and its relation with demographic and clinico-pathological factors were analyzed. This retrospective study was approved by the local scientific and ethical committee.
2.1. Statistical Analysis
Patients’ baseline characteristics, disease and treatment factors were summarized using descriptive statistics. The categorical parameters were compared using two-sided Pearson’s χ2 test or Fisher’s exact test, as appropriate. The overall survival (OS) time was defined as the period from the diagnosis until death of any cause or until the date of the last follow-up, at which data point was censored. All summary statistics on time-to-event variables were estimated according to the Kaplan- Meier method and compared using the Log-rank or Breslow test. SPSS software (version 21.0) was used for statistical analysis. A P value<0.05 was considered significant.
3. Results
3.1. Patient, Tumor and Treatment Characteristics
Of 88 included patients, 56 were male (64%) and 32 were female (36%) with mean age of 46 years (13 to 89 years). Half of the patients (50% of all) had normal range body mass index (BMI). According to primary tumor and lymph node stage, most cases were T1/T2 (70.5%) and N2/N3 (58%) respectively. Eight patients had distant metastasis at presentation and lungs were the most common metastatic site. Induction chemotherapy plus concurrent chemo-radiation was the most common treatment approach. The patient, tumor and treatment characteristics are detailed in Table 1.
| Characteristics | No. (%) |
|---|---|
| Age, y | |
| ≤ 50 | 53 (60) |
| > 50 | 35 (40) |
| Sex | |
| Female | 32 (36) |
| Male | 56 (64) |
| BMI classification | |
| Below Normal | 10 (11) |
| Normal | 44 (50) |
| Above Normal | 34 (39) |
| T-stage | |
| T1/T2 | 62 (70.5) |
| T3/T4 | 26 (29.5) |
| N-stage | |
| N0/N1 | 37 (42) |
| N2/N3 | 51 (58) |
| M-stage | |
| M0 | 80 (91) |
| M1 | 8 (9) |
| Site of Metastasis | |
| Lung | 4 (50) |
| Bone | 2 (25) |
| Other | 2 (25) |
| Treatment | |
| Chemo then ChemoRT | 76 (86) |
| Chemo then RT | 7 (8) |
| ChemoRT | 4 (5) |
| Missing data | 1 (1) |
The Patient, Tumor and Treatment Characteristics
3.2. Survival Analysis
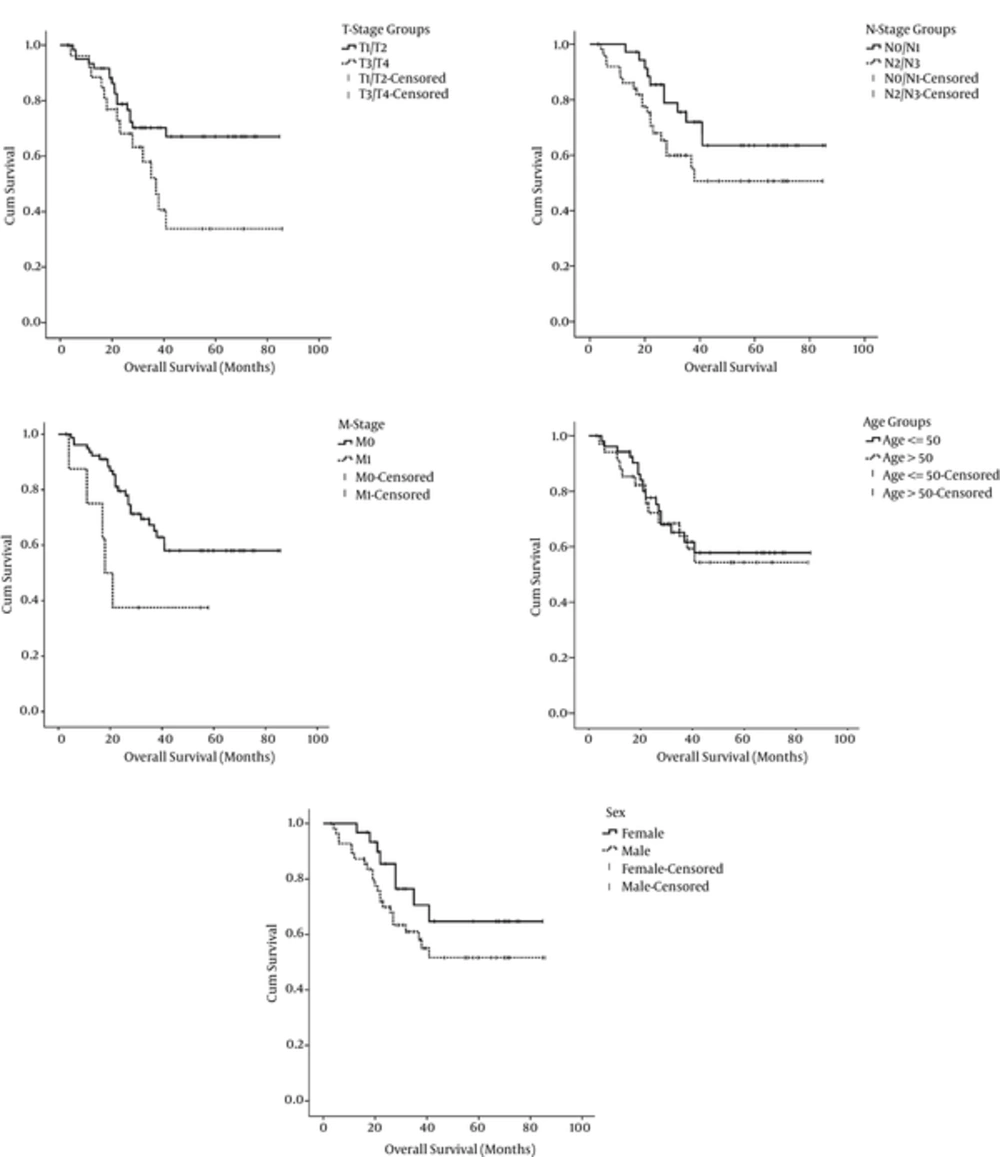
With a median follow-up period of 31 months (range: 3 to 86 months), overall survivals of the whole patients and those without distant metastasis were 64% and 67%, respectively. Tumor stage (T-stage) and presence or absence of distant metastasis at presentation were the only parameters that significantly influenced the patients’ OS. Mean OS of patients with T1/T2 tumors was higher than patients with T2/T3 tumors and this difference was statistically significant (64 months vs 46 months respectively: P = 0.04). Patients with established distant metastasis at presentation had significantly lower mean OS than patients without metastasis (31 months vs 60 months respectively: P = 0.032). Although the differences between node stage and sex subgroups in terms of OS were not statistically significant, propensity toward statistical significance were observed (P = 0.064 and P = 0.097 respectively). Age and Body Mass Index (BMI) classifications had no significant correlations with the overall survival. The correlation among survival, patient, and tumor characteristics are detailed in Table 2 and Figure 1.
| No | Mean OS, mo | P Value | |
|---|---|---|---|
| Sex | 0.097 | ||
| Female | 32 | 65 | |
| Male | 55 | 55 | |
| Age, y | 0.783 | ||
| ≤ 50 | 53 | 60 | |
| > 50 | 34 | 57 | |
| BMI Classification | 0.719 | ||
| Below Normal | 10 | 49 | |
| Normal | 43 | 56 | |
| Above Normal | 34 | 62 | |
| T-stage | 0.04 | ||
| T1/T2 | 61 | 64 | |
| T3/T4 | 26 | 46 | |
| N-stage | 0.064 | ||
| N0/N1 | 36 | 65 | |
| N2/N3 | 51 | 53 | |
| M-stage | 0.032 | ||
| M0 | 79 | 60 | |
| M1 | 8 | 31 |
Correlation Among Survival, Patient and Tumor Characteristics
4. Discussion
In the present study, patients’ demographic characteristics like age and sex were similar to the ones in other studies (both local and in other geographic regions of the world) in terms of male predominance and younger age at diagnosis compared with other head and neck carcinomas (4, 6, 10-12). Most patients of our study had T1/T2 and N2/N3 disease at presentation and these findings are in line with some other reports (4, 11). The rate of distant metastasis at the time of diagnosis (9% of all cases) was also similar to the ones observed in other patient populations (11). With a median follow-up period of 31 months, OS of our patients without established distant metastasis was 67%. This survival result is inferior in comparison with some other reports. Liu et al. evaluated 83 patients with non-metastatic nasopharyngeal carcinoma treated with intensity modulated radiation therapy (IMRT) with or without chemotherapy and analyzed the factors influencing their outcome. Three-year OS of the patients was 82% and stage of disease, N-classification and cumulative dose to primary tumor were significant prognostic factors for overall survival (10). In another study in China, Wang et al. showed three-year overall survivals of 83% and 88% among Uyghur and Han patients, respectively. All 181 patients had non-metastatic nasopharyngeal carcinoma mainly stage 3/4 disease and treated by IMRT with or without systemic chemotherapy (4). An explanation for the inferior survival rate of our patients compared with the above-mentioned studies may be the different races and geographic regions of the patient populations. Another explanation may be different treatment approaches to the patients as nearly all our patients (95%) received induction chemotherapy before definitive local therapy. With a more similar treatment approach and patient population to our study, Fountzilas et al. in a randomized phase II trial reported a three-year overall survival of 67% for the patients of their investigational arm. 141 patients with non-metastatic nasopharyngeal carcinoma were randomly assigned to investigational (received induction chemotherapy plus concurrent chemo-radiation) and control (received only concurrent chemo-radiation) arms. The induction chemotherapy regimen consisted of three cycles of cisplatin plus epirubicin plus paclitaxel and concurrent chemotherapy was weekly cisplatin. Patients of the induction chemotherapy arm had mainly T1/T2 and N2/N3 disease and their overall survival was similar to the ones observed in our patients (13).
4.1. Conclusions
In the present study, T-stage and presence or absence of distant metastasis at presentation were demonstrated to be significantly associated with overall survival. Although the differences between node stage and sex subgroups in terms of OS were not statistically significant, trends toward significance were observed. These findings are consistent with results of most other studies (4, 10, 14). In conclusion, our study provides some evidence on the outcome of Iranian patients with nasopharyngeal cancer and factors influencing it.