1. Introduction
Human melanoma, a malignant tumor of melanocytes, which is characterized by rapid growth (1, 2) and a higher mortality rate (2) rather than any other cancer, remains as one of the most treatment-refractory malignancies (1).
A major challenge facing chemotherapy of melanoma that has limited treatment efficacy is resistance to conventional chemotherapy regimens (3, 4). Cisplatin is recommended for melanoma patients routinely (5). Resistance to cisplatin is not completely understood; however, it is thought to occur through many different mechanisms (6, 7). Hyperthermia (also referred to as thermotherapy) is one of the most promising new multidisciplinary approaches to cancer therapy (8-10). While high temperature hyperthermia (using temperature over 50°C) can be considered as a single modality treatment, hyperthermia typically serves as an adjuvant technique that utilizes subletal heat (40 - 43°C) to increase the therapeutic effects of cancer treatment modalities such as radiotherapy (9, 11, 12), chemotherapy (12, 13) and immunotherapy (8). The combination of hyperthermia with such modalities has been applied in various types of malignancies, including melanoma (14), sarcoma, cancer of the head and neck, brain, lung, esophagus, breast, rectum, liver, appendix, cervix, and mesothelioma.
Applications of thermal therapies have been considered as numerous; because most thermal approaches are minimal or non-invasive, relatively simple to perform, and have the potential of treating embedded tumors in vital organs where surgical resection is not feasible (15).
Although the cellular and molecular basis of hyperthermia have been thoroughly reviewed by numerous researchers (16-21), the molecular mechanisms underlying the ability of hyperthermia to increase sensitivity to cytotoxic agents are not fully understood. Mild hyperthermia (39 - 43°C) can increase cellular sensitivity to anticancer drugs by accelerating the primary step in a drug’s efficacy which is increasing the intracellular drug concentration (22) by overcoming drug resistance and increasing the blood flow. This may reduce the required effective dose of the anti-cancer drug. The effect of hyperthermia depends on both temperature and exposure time (23, 24).
Recent developments in nanobiotechnology have brought a large number of new modalities for cancer treatment (22, 25). Obviously, novel therapy approaches which improve cancer treatment outcomes while minimizing toxicity are still needed.
Some of metallic nanoparticles can be made a resonant response to magnetic field, with advantageous consequences related to the transfer of energy to the particles (25, 26), so this leads to its use as a hyperthermic agent. Among metallic nanoprticles, gold nanoparticles are promising tools due to their unique electronic, optical, thermal, chemical, and biological properties and their potential catalytic applications in various fields such as biology, medicine, physics, chemistry and other interdisciplinary fields (27-29). Their medical usage in human body has been approved by the FDA (29). It has been reported that human cells can take up GNPs without cytotoxic effects (30). GNPs have been shown to have antiangiogenic properties in solid tumors and appear to induce apoptosis in B chronic lymphocytic leukemia cells (31). Most studies on GNPs based cancer therapy have used photothermal therapy by laser GNPs, which have been shown to improve the therapeutic efficacy of kV X-Ray (32, 33) and electron-beam irradiation (34). They can also serve as a type of “molecular surgery” or “thermal scalpel “for the removal of Alzheimer,s amyeloid deposits by using weak MWs (26).
We examined the heat-enhancing effect and permeability-enhancement of gold nanoparticles in combination with cisplatin in the presence of MW exposure on MM200 melanoma cell line which may have therapeutic potentials for the treatment of melanoma.
2. Methods
2.1. Preparation of GNPs
To prepare the required GNPs, the following steps were taken (35):
A 50 mL solution was prepared by dissolving HAUCL4 (purchased from Sigma- Fluka CO.) in water with a concentration of 0.01 M and using a phosphate buffer system the ion strength and the pH of the prepared solution reached to 0.005 M and 7.8, respectively. A 50 mL non-aqueous phase (Toluene) containing sodium tetraborohydrate, sodium borohydride (NabH4); purchased from Sigma-Aldrich; (Lot no. 247677) in 0.02 M concentration was prepared separately. Both phases were mixed together and stirred severely. The organic phase was separated and the solvent was removed by a rotary device in low pressure at 50°C. GNPs, gathered at the bottom of the container, were dispersed in a phosphate buffer solution with 0.005 M ion strength and a pH of 7.6; hence a homogenous solution was produced. Transparency and red color were the two obvious characteristics of the solution.
2.2. Determining the MW Irradiation Time
To determine an appropriate time for inducing hyperthermia through MWs, 6-well plates containing the cells suspended in 5 mL culture medium were exposed to 2450 MHz irradiation (Using a Panasonic MW generator NN-ST565W). In separate triplet measurements, the changes of temperatures were recorded at 5 minute intervals by a digital thermometer (APPA51) equipped with K-type thermocouple and 0.1°C sensitivity. Then the curve of temperature variations versus exposure time was drawn. The exposure times for the cell samples to reach 41 and 43°C were determined as 25 and 30 minutes, respectively.
The effect of GNPs on temperature enhancement was determined at concentrations of 0 .013, 0.019 and 0.026 mg/mL after 25 and 30 minutes of MW exposure. It can change the temperature between 44.6°C to 46.1°C.
2.3. Cell line and Cell Culture
MM200 cell line was cultured in DMEM (purchased from Gibco Life Technologies) supplemented with 20% w/v FBS (purchased from Gibco Life Technologies), 100 µg/mL of streptomycin (purchased from Jaberebne hiian, Inc), 100 IU/mL of penicillin (purchased from Jaberebne hiian, Inc), and 2 µM of L-glutamine (purchased from Gibco Life Technologies). The cells were then adapted with RPMI 1640 medium supplemented with 10% FBS, penicillin (100 IU/mL), and streptomycin (100 µg /mL) in a 75-cm2 flask and incubated at 37°C with 95% (v/v) air and 5% (v/v) CO2 in a humidified atmosphere. The cells were harvested by treating the flasks with trypsin-EDTA for 3 min, when the cells were in exponential growth phase. Then they were washed and counted, and a cell suspension of a density of 6 × 103 cells/well was prepared. An aliquot was placed in each well of a 96-well plate in a total volume of 100 µL. Afterwards, the cell samples were conducted to the designed treatments.
2.4. Treatment Conditions and Experimental Groups
To investigate the potential effect of hyperthermia on cell growth and survival, cell viability was assessed by the MTT assay. In brief, the cells were seeded into 6-well plates at a density of 5 × 105 cells/well and were exposed to MW exposure for 25 and 30 minutes. Then they were allowed to incubate for the hours at 37°C. For combination therapy with drug and GNPs, following chemical agent treatment, the cells were exposed to hyperthermic temperature (and were then allowed to incubate for 24 hours at 37°C).
The antiproliferative activity of single therapy was assessed in monolayer culture conditions, with cells grown in 96-well plates at a density of 6 × 103 cells/well for 3 hours. Then the cells were cultured in media containing various concentrations (0.5, 1.0, 1.25, 2.5, 5.0, 10.0, 20.0, 30.0, 40.0 and 50.0 µg/mL) of cisplatin for 24 hours (N = 5) (5, 36), or with various concentrations of GNPs for 24 hours (32). To evaluate the anti proliferative effects of the combined treatment, the following exposure to different concentrations of cisplatin (0.125, 0.25, 0.5, 1.0 mM), GNPs were added to each well and the plates were incubated for 24 hours at 37°C.
Experimental groups:
A) The control group with no treatment
B) Group 2: Cell incubation in the presence of GNPs
C) Group 3: Chemotherapy (Cell incubation in the presence of cisplatin)
D) Group 4: Chemotherapy in the presence of GNPs
E) Group 5: Hyperthermia (at 25 and 30 min. exposure by MW) in the absence of GNPs
F) Group 6: Hyperthermia in the presence of GNPs
G) Group 7: Hyperthermia in the presence of cisplatin
H) Group 8: Hyperthermia and chemotherapy in the presence of GNPs
2.5. Cell Viability Assay
The viability of melanoma cells treated with chemical agents was determined via the MTT assay, and linearity between cell numbers and OD values was established. After applying treatments, the culture medium was replaced with serum-free medium containing 5 mg/mL MTT, and the cultures were incubated for an additional 4 hours. After that, the medium was aspirated from each well as far as possible and 200 µL of DMSO was added to each well to dissolve the formazan crystals. The plates were shaken at room temperature for 10 minutes and read immediately at 545 nm using STAT FAX 2100 microplate reader. Wells containing only RPMI and cells were used as controls. All experiments were performed on three separate cultures.
2.6. Data Analysis
The cell response was assessed by one-way analysis of variance (ANOVA), and Tukey test with the SPSS software version 16. The data are shown as mean ± SD. P values less than 0.05 was considered as significant.
The IC50 value (drug concentration producing 50% inhibition of cell growth, by a 24-h incubating with the drug compared with untreated control cells) was calculated for single therapy by cisplatin alone. Combined therapy was evaluated using the cisplatin dose response points combined with GNPs at 37°C at each time point. Relative lethal synergism (RLS) (35) also was calculated to compare the data. That is equal to the ratio of percentage of actual cell viability versus cell death estimated based on the impact of any therapeutic agent
3. Results
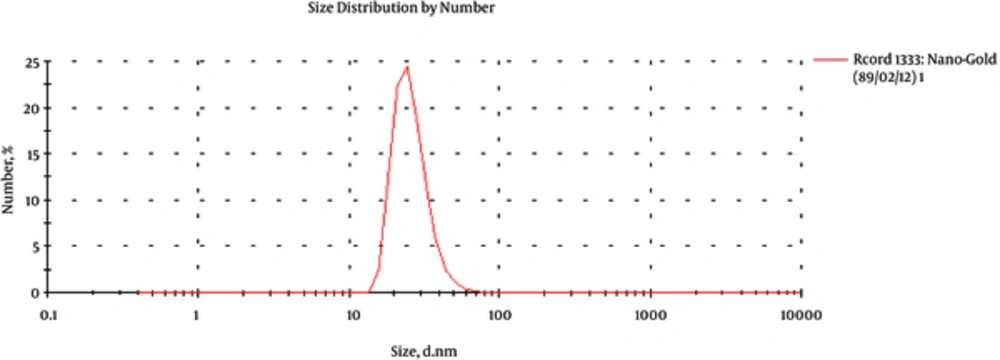
3.1. Size Distribution of GNPs
Size range of spherical GNPs was characterized by a Zeta-Sizer (nano-ZS, Malvern, England). Observation using Zeta-Sizer showed that GNPs were close to 57 nm (Figure 1).
3.2. Cytotoxicity of the Agents
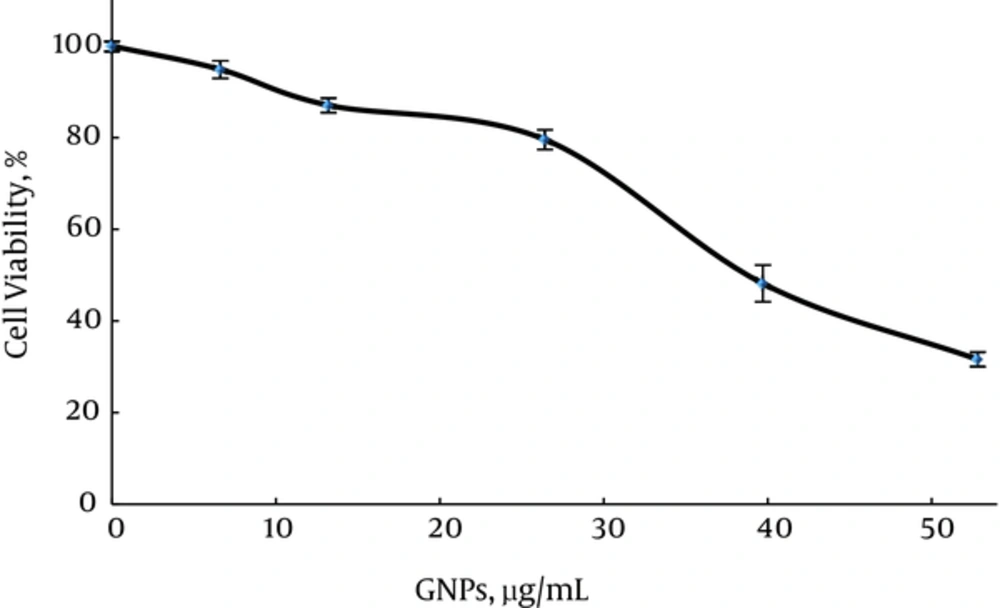
Firstly, in group 2, the cytotoxicity of GNPs was determined according to the percentage of viable cells in varying concentrations of GNPs without MW radiation and cisplatin compared to the control. This study was also performed to determine the highest concentration of GNPs that does not affect cell viability. In Figure 2, it can be seen that a significant decrease in cell viability was observed when cells were incubated with 26.4 µg/mL GNPs (survival rate = (79.5 ± 2.1) %). This effect increased as GNPs concentration increased to 52.8 µg/mL (survival rate = (32.6 ± 1.5) %). In Figure 2, GNPs with 13.2 µg/ml did not show any significant difference compared to GNPs with 6.6 µg/mL (P ≥ 0.576) and with 26.4 µg/mL (P = 0.947).
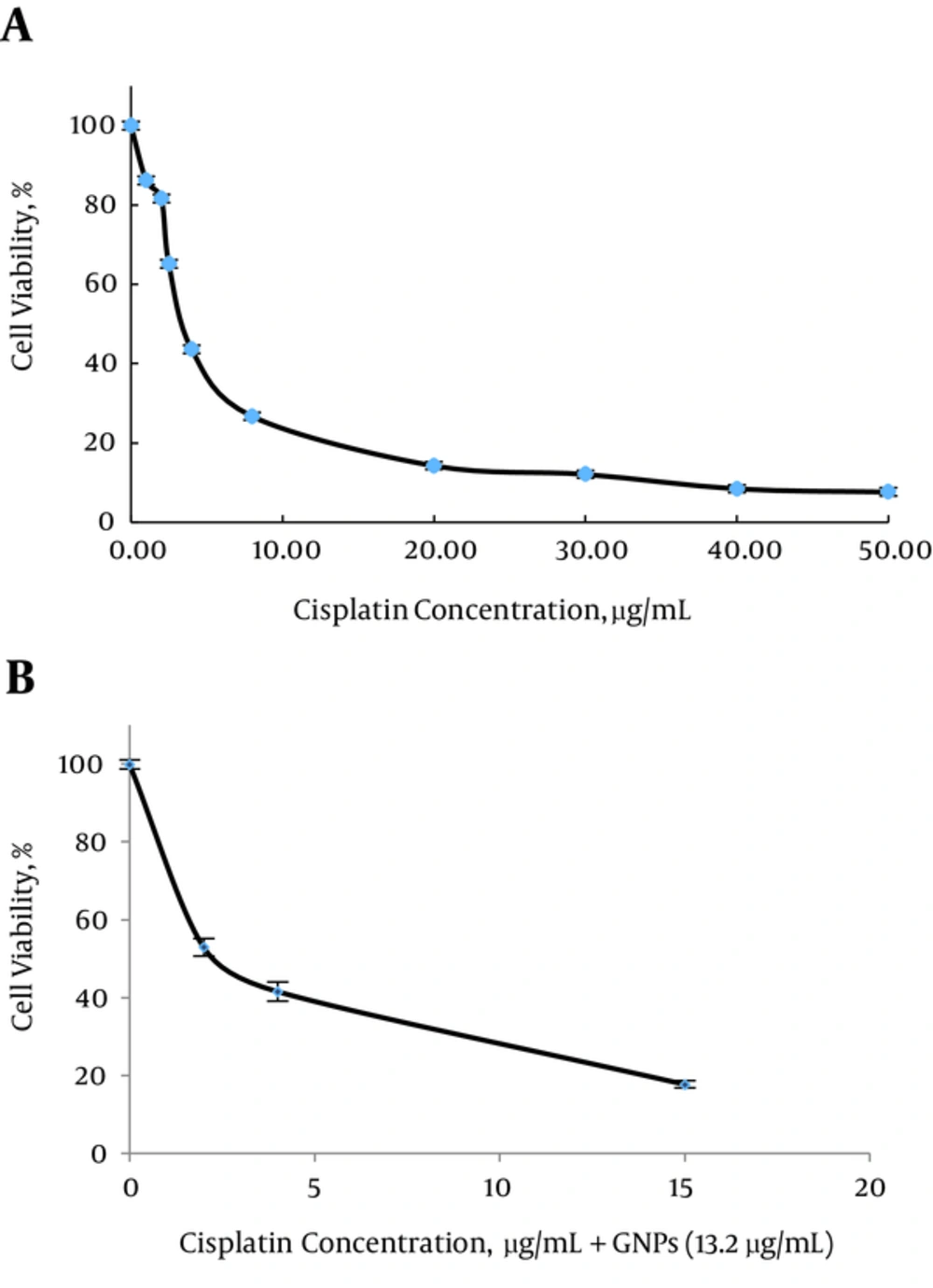
In group 4 GNPs concentration was selected as 13.2 µg/mL (Survival rate = 86.9 ± 1.5%) to examine the effect of GNP plus cisplatin on growth inhibition (Figure 3B).
Our results showed, IC50 of cisplatin in the presence of GNPs significantly lower than cisplatin alone in MM200 cells (P < 0.001, Table 1). IC50 of cisplatin was determined as 4 µg/mL (Figure 2A), which reduced to half, in the presence of GNPs.
| Treatment Group | IC50, µg/mL |
|---|---|
| Cisplatin | 4 |
| Cisplatin + GNPs | 2 |
In the following experiments, 2 µg/mL (lower than IC50; Survival rate = (81.6 ± 3.04) %) of cisplatin was used to examine the effect of GNPs plus hyperthermia and cisplatin on growth inhibition.
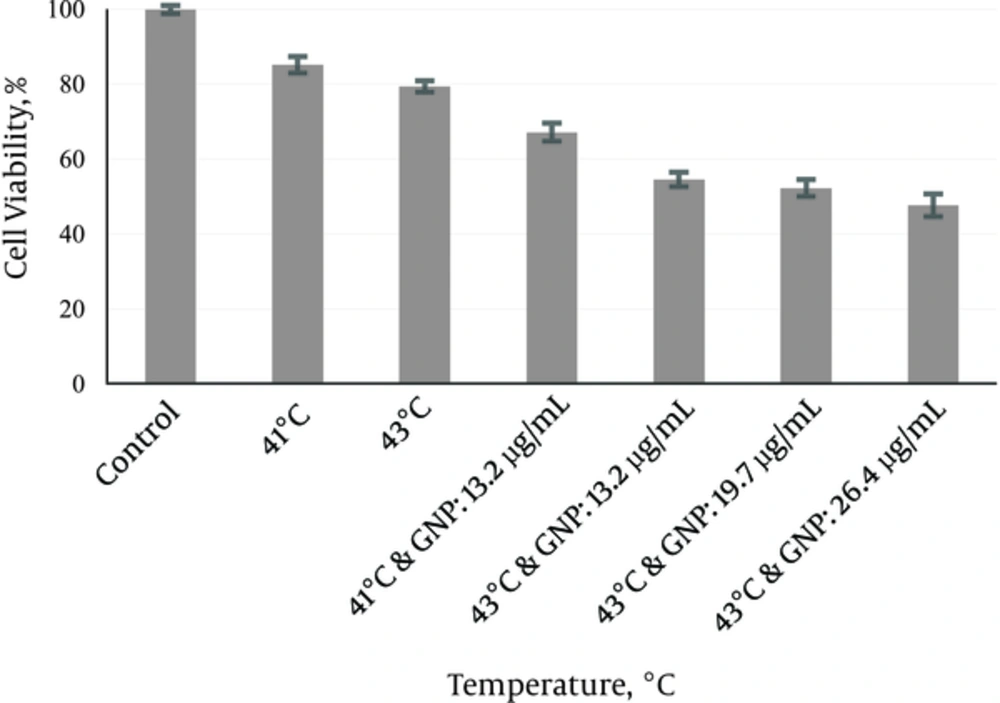
In group 5, MW exposure was applied in the absence of GNPs at 41°C and 43°C. After 24 hours, the mean survival rate was determined (85.17 ± 2.2) % and (79.36 ± 1.4) %) (Figure 4). According to Figure 4, MW exposure at 41°C and 43°C did not show any significant differences relative to each other (P = 0.959) but it showed significant differences relative to control (P < 0.001).
In group 6, the effects of GNPs (13.2 µg/mL) were applied on cell survival fractions at different dose of MW (Figure 4). At 41°C and 43°C the enhanced cytotoxicity of GNPs (13.2 µg/mL) showed significant differences relative to hyperthermia and GNPs alone (P < 0.001). Concentrations of GNPs ranging from 13.2 to 26.4 µg/mL (at 43°C) were used to observe the effects of GNPs concentration on the enhancement of MW hyperthermia cytotoxicity on the MM200 cells. Concentration-response of cytotoxicity effect of GNP was shown in Figure 4. As shown in Figure 4, MW hyperthermia at 43°C in the presence of different concentrations of GNPs did not show any significant differences in survival rate.
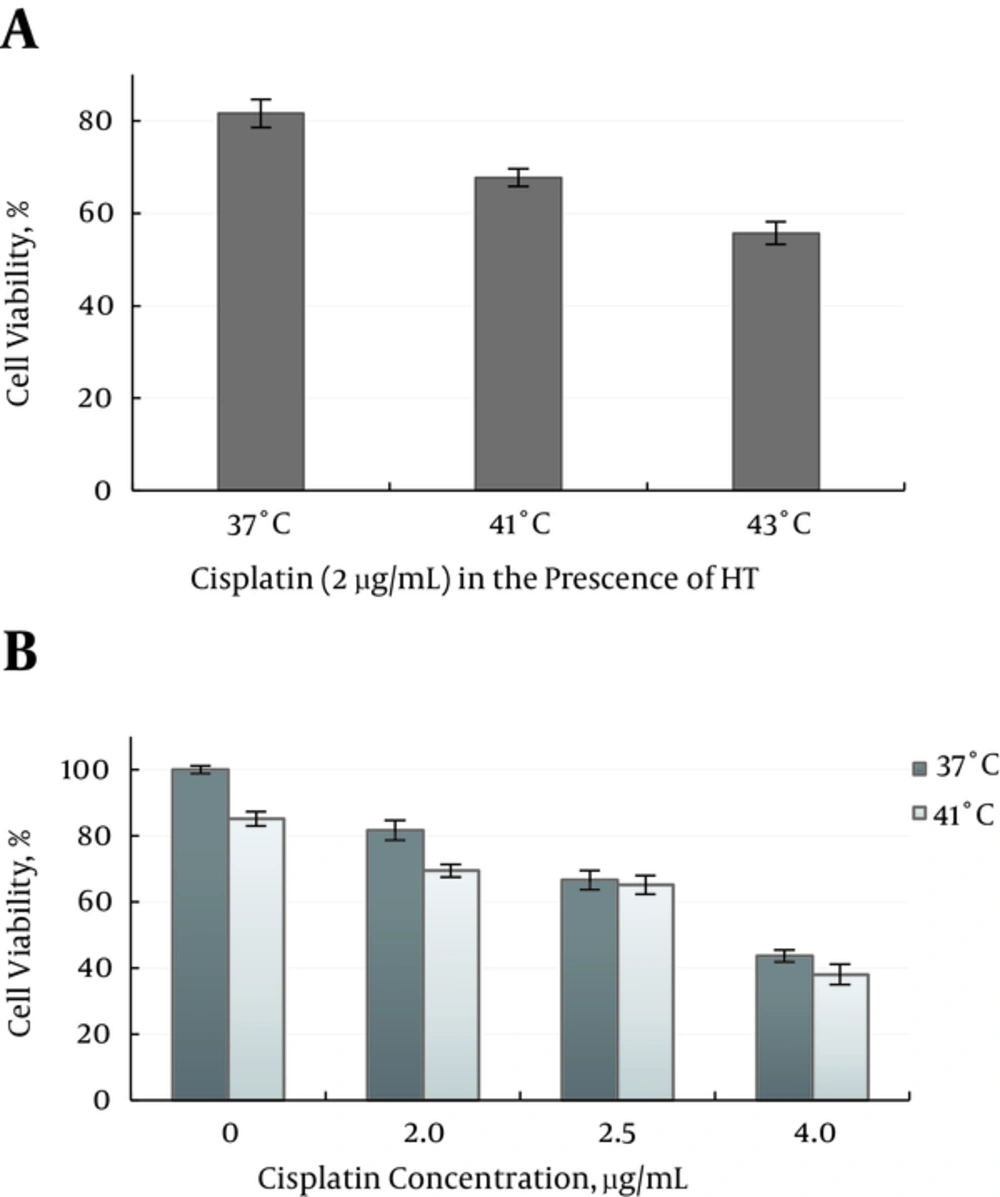
In group 7, the cisplatin cytotoxicity at the similar dosage (2 μg/mL), were determined at 37°C, 41°C and 43°C Figure 5A. Results showed, there is no significant enhancement in cisplatin cytotoxicity on the MM200 cells relative to 37°C (P ≥ 0.547).
At 41°C the effects of cisplatin concentration (2 - 4 µg/mL) were applied on cell survival fractions, no concentration-response of cytotoxicity effect of cisplatin was achieved Figure 5B.
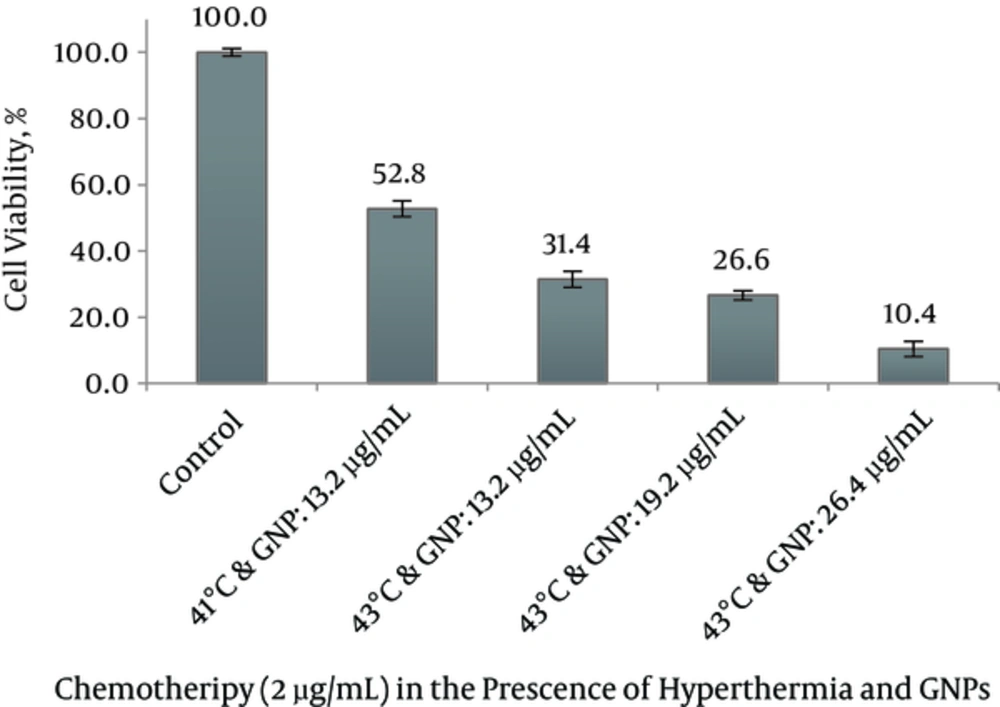
The combined treatment of MM200 cells with 2 μg/mL cisplatin plus 13.2 µg/mL GNP in group 8 induced a 2.55 fold increase in cisplatin cytotoxicity at 43°C and 1.45 fold increases at 41°C relative to 37°C (Figure 6). The survival rates in these groups were recorded as 31.4 ± 2.4 % and 52.8 ± 2.4%. Combined treatment of 2 μg/mL cisplatin plus 13.2 µg/mL GNP at 43°C showed significant difference relative to control, treatment with chemotherapy and GNPs alone, chemotherapy and GNPs in the presence of hyperthermia (P < 0.001) but combined treatment of 2 μg/mL cisplatin plus 13.2 µg/mL GNP at 41°C showed no significant difference relative to chemotherapy in the presence of GNPs (P = 1.000).
Notably, the highest degree of growth inhibition was observed with 26.4 µg/mL GNPs in combination with cisplatin in the 43°C (survival rate = 10.4 ± 2.3 %). Addition of 26.4 µg/mL GNPs to 2 μg/mL cisplatin at 43°C provided at least the same level of cytotoxicity as a 20-fold higher concentration of cisplatin alone in MM200 cell line.
So it can be seen that co-treatment with moderate hyperthermia enhances the synergistic effect of the combined cisplatin and GNPs treatment. In addition, a dose-dependent enhancement induced cytotoxicity of GNP was observed when MM200 cells were treated with a cisplatin at 37°C and in combination with MW exposure (Figure 6).
Relative lethal synergism (RLS) in combination therapy with cisplatin and hyperthermia in the presence of GNPs to predict the actual and estimated rates of cell death calculated (shown in Table 2). Maximum RLSs of GNP were obtained as 2.5, 2.4 and 2.1 for the hyperthermia, chemotherapy and hyperthermia with chemotherapy, respectively.
| Treatment | GNPs, µg/mL | The Actual Percentage of Cell Death | The Estimated Percentage of Cell Death | RLS (Relative Lethal Synergism) |
|---|---|---|---|---|
| Hyperthermia | 13.2 | 45.4 | 20.6 | 2.1 |
| 19.7 | 47.7 | 20.6 | 2.3 | |
| 26.4 | 52.2 | 20.6 | 2.5 | |
| Chemotherapy | 13.2 | 47.1 | 19 | 2.4 |
| Chemotherapy + Hyperthermia | 13.2 | .668 | 44.3 | 1.5 |
| 19.7 | .473 | 44.3 | 1.6 | |
| 26.4 | 89.6 | 44.3 | 2.1 |
4. Discussion
In this study, the anti-proliferative potentials of cisplatin and GNPs in combination with hyperthermia were evaluated in a human melanoma MM200 cell line. A “gold standard” metabolic assay for cytotoxicity is the MTT assay (30). As the recorded data in Table 1 shows, there is a synergistic effect between cisplatin and GNPs without hyperthermia, and cisplatin IC50 reduced by half in the presence of GNPs. This could be due to the facilitated entrance of cisplatin in the presence of GNPs. Reduced cisplatin uptake by the platinum resistant cells may be associated with several factors such as defective endocytosis and MDR (7). About the results achieved, one assumption is entrance of cisplatin in the presence of GNPs that facilitated. It has shown GNPs with a diameter less than 100 nm enhance endocytosis and permeability and retention effect (EPR) (37-39). This mechanism has often resulted in 10-fold or greater drug delivery to a tumor over conventional chemotherapy (40). GNPs are taken up by cells with receptor–mediated endocytosis (41). Melanoma cells take up nanoparticles via melanocortin type-1 receptor–mediated endocytosis (42). Also in vivo studies on GNPs uptake have shown dependence on size, shape, zeta potential and exposure time (43, 44). For example, Hela cells indicated that GNPs with 50 nm diameter have maximal endocytosis (33). Chen et al showed that mammalian cells take up GNPs (nears 50 nm) at higher rates in comparison to both smaller and larger particles in the range of 3 - 100 nm (45) and compared with nanorod (30). The effect of this optimal size (50 nm) was called “wrapping effect”, which explains how a cellular membrane encloses nanoparticles and two factors contribute in this, free energy that results from ligand-receptor interaction, and receptor diffusion kinetics onto the wrapping sites on the cellular membrane (46).
Moreover, the size reduction of particles, increases its surface area relative to the volume, and enables it more reactive on self and to biological components and then their interaction with biological components increases (30).
Thus, GNPs may also influence the protein binding profile of cell membrane proteins, change their conformation and affect drug entrance into the cells and or influence DNA repair processes that are important ways in drug resistance to the platinum, whereas the selective and irreversible binding of GNPs with cell components have been considered in various studies, for example the anti-angiogenic and radio-sensitizing effect of GNPs which is related to the interaction of GNPs with proteins (30, 31, 33).
Based on figure 2C, MM200 cell line shows no significant decrease in cell survival in certain GNPs concentrations. Cytotoxicity of GNPs also depends on the type of cells used, zeta potential and the localization of GNPs in cell components. Chan et al. indicated that GNPs of 3, 5, 50, and 100 nm do not show any toxic effects on mice (45). However, 33 nm citrate-capped gold nanospheres were seen to be noncytotoxic to baby hamster kidney and human hepatocellular liver carcinoma cells, but it shows cytotoxicity to a human carcinoma lung cell line at certain concentrations (47).
In this study cytotoxicity of cisplatin alone at 41°C and 43°C did not show any enhancement compared to 37°C (at the same concentration) also at 41°C any cytotoxicity enhancement in response to increase in concentration was not observed. But in the presence of GNPs at the same temperature, cytotoxicity increased to 1.4 - 2.5 folds. According to figure 5, cell viability of cisplatin with a dosage of 2 µg/mL in the presence of GNPs and MW exposure was reduced to at least to the same level as a 35 µg/mL dose of cisplatin alone. This result shows that the addition of GNPs in the presence of MW exposure may help for a reduction of the cisplatin dose needed to achieve the same cytotoxic effect. It was considered that this consequence might be attributed to a higher entrance of cisplatin into the cell which has been facilitated in the presence of GNPs and thus enhancement of the hyperthermia (HT) efficacy with quicker increasing temperature and subsequent intensify drug cytotoxicity. This result may be also related to RLS consequence (Table 2). It was suggested that cisplatin may play the most important role in intensifying cytotoxicity in this combination therapy, whereas selection of GNPs and MW has an assistant role (facilitated entrance and overcome obstacles in cell membrane and enhancement prepared temperature and so overcome to sublethal repair resistance). While many studies have considered.
It has been suggested that hyperthermia up to 43°C combined with chemotherapy produces a “more than additive” effect (1), compared to the higher concentrations of chemotherapeutic agents (48).
Cisplatin interacts with DNA to form DNA intra- or inter-strand crosslinks and/or DNA-protein crosslinks, thus induces cytotoxicity (49). Cisplatin in combination with hyperthermia causes an increase in intracellular drug accumulation and induced DNA-platinum adducts (18). An inhibition of DNA repair bases following increased temperature was noted in cisplatin-treated cells (18).
It is important to note that the results mentioned above depend on the temperature and exposure time of hyperthermia (27). Meanwhile in sublethal temperatures, cells can acquire their normal cellular metabolism. These cells leading to acquire thermal tolerance due to heat shock protein expression (1) especially in the 70 KD (50, 51). Heat shock proteins may be associated with DNA repair enzymes, such ‘mismatch repair’ or the ‘nuclear excision repair proteins’ so protect cells (16, 18).
4.1. Conclusions
Based on these findings we conclude that the combination of both GNPs and MW based hyperthermia is effective in improving cisplatin therapy of melanoma cells. Moreover, adding MW to combination of GNPs and cisplatin increased its anti-proliferative activities compared to all treatments given separately, which expected as a result of temperature increasing on GNPs. It is expected that the combination of cisplatin, GNPs and MW based hyperthermia offer a great promise in therapeutic selectively of melanoma. Taken together, further evaluations could be recommended to detect the details of multi-mechanism (resistance mechanisms and cell sensitization) underlying each of the above agents individually or in combinational.