1. Context
According to global statistics, breast cancer has known as the most common cancer in women around the world (1). It has reported as the most common cancer and the second leading cause of cancer deaths among Iranians women (2). Breast cancer was also the most frequent cancer among Iranian women with incidence rate of 25 per 100,000 (3). Diabetes, metabolic syndrome and breast cancer were the main problems in developed and developing countries based on people lifestyle (4). The results of a study aimed at investigating the diabetes impact on breast cancer risk, reported that, 40% of deaths in the first 5 years after breast cancer have occurred in women with diabetes (5).
Studies have shown there would be a strong association between diabetes and cancer (6). The use of metformin might lead to reduction of liver cancer, pancreas cancer, colon cancer, and breast cancer incidence. It could also reduce the deaths from liver and breast cancer (7). Metformin, as a drug for treatment of diabetes, has approved in Britain at 1958, 1972 in Canada and 1995 in the United States (8). It has also considered as the only biguanide for type 2 diabetic patients with the lowest risk of side effects (9). Metformin was one of the most common anti-diabetic medicines which plenty evidences have suggested its potential impacts as an anti-cancer drug (10).
In this regard, results have shown that women with diabetes with long-term use of metformin were less prone to breast cancer. Of course, this finding has not approved in relation to short-term use of metformin, sulfonylureas and other anti-diabetic drugs (11). Totally, results have shown that breast cancer patients who have used metformin had better results than those who has not used (12). On the other hand, the results of a meta-analysis which has conducted to evaluate the use of metformin in breast cancer risk has shown the protective effect among the diabetic women, and this finding has particularly emphasized further in long-term use (13). According to the above findings, Results of a meta-analysis have shown a 31% reduction in incidence of cancer and cancer deaths in users of metformin in comparison with other diabetes medicines (14). Also the increased risk of cancer-related death in diabetic patients who have used sulfonylureas and exogenous insulin was much more significant in comparison with metformin users (15). Clinical evidences have suggested that metformin could prevent proliferation and growth of certain types of cancers, such as breast cancer, even in absence of diabetes (16). According to the above results about the efficacy of metformin in treatment and prevention of breast cancer death; in comparison with other medicines such as sulfonylureas, it would be necessary to perform a meta-analysis in related research of metformin effects on prevention and treatment of breast cancer The results of this study could be the basis for making beneficial therapeutic purposes in prevention, treatment and reducing the effects of breast cancer, such as breast cancer mortality.
2. Evidence Acquisition
We have performed a systematic review of best available evidences using Cochrane Collaboration guidelines for systematic review of interventions. Our structured question for this review was as follows in Table 1.
| Components | |
|---|---|
| Population | Women with type 2 diabetes |
| Intervention | Metformin |
| Comparator | Sulfonylurea |
| Outcome | Breast Cancer Risk, Mortality for Breast Cancer |
| Type of studies | Randomized Controlled Trials and observational studies |
Components of Structured Question
2.1. Search Strategy and Study Selection
We have searched the most important and appropriate electronic medical databases including MEDLINE, PubMed, Cochrane library, Science Direct, Trip, Google Scholar, Institute of Scientific Information (ISI), SCOPUS and EMBASE as well as relevant websites have searched without time limitation up to June 2015. The MeSH system has used by and and or between words of the same meaning. Metformin, sulfonylurea, Breast, cancer and diabetes were the key words. Extracted articles have organized in Endnote software. After deleting duplicate articles, two reviewers have assessed the titles and abstracts of search results independently and selected relevant studies according to our main question (Table 1). The articles that have deemed to be irrelevant to the research objectives were excluded. Then, the full texts of the selected articles have gathered. Those articles that have not possessed the inclusion criteria have excluded. Two readers have determined the eligibility of each article for inclusion independently. Discrepancies between readers have resolved in conference.
2.2. Inclusion and Exclusion Criteria
The inclusion criteria were the following: 1, studies on women with diabetes mellitus that reported data on exposure to metformin therapy, in comparison with Sulfonylurea, and breast cancer incidence or mortality; 2, studies presenting the relative risk (RR), odd ratio (OR) or hazard ratio (HR) estimation, 95% confidence intervals (CIs) or P-value, size of baseline samples, or other information that could help to interpret the results; and 3, studies written in English language.
The publications have excluded if they have met any of the following criteria: 1, therapy with medicines other than metformin; 2, other types of cancer; 3, lack of the focus on association between metformin and breast cancer; 4, basic or animal research; 5, review; and 6, absence of relevant data; 7, duplicate articles that have up-to-date versions available. Studies that have not published as full reports have excluded.
2.3. Quality Assessment
Two authors have assessed the quality of each study based on the critical appraisal skills programme (CASP) checklist independently. All the differences have resolved by discussion. The quality of these studies has evaluated by triple sections examination: were the results of these trials valid? (Section A), What were the results? (Section B), and Would the results help locally? (Section C).
2.4. Data Collection
We have extracted details of study design, country, publication year, intervention and comparator groups, adjustment and stratification variables, sample size, event and non-event, has reported outcome measurements and the 95% CI. Disagreements have settled by consensus.
2.5. Statistical Methods
We have analyzed data using Review Manager Software (RevMan version 5.1.7; Nordic Cochrane Centre, The Cochrane Collaboration, 2011) and Comprehensive Meta-Analysis Software. The summary RR for exposure to metformin or sulfonylurea was the measure of interest. Analyses have performed for specific reference therapies and breast cancer risk on condition that the corresponding estimation has reported by at least two studies. The I² statistic has used for evaluation of heterogeneity. Heterogeneity has measured based on the following:
1, I2 < 40 = the heterogeneity would be acceptable.
2, 40 < I2 < 70 = the heterogeneity would be average.
3, I2 > 70 = the heterogeneity would be high.
An I2 value of 40% or more indicated substantial heterogeneity and it has meant the results of meta-analysis had high heterogeneity and it was not valid (17). We have pooled the original estimation by using both the fixed-effects model and the random-effects model have proposed by Mantel-Haenzel. In cases of differences in statistical significance of the effect estimation between the two models, we have reported both results; otherwise, we have reported results of the random-effects model. Random model has applied when the heterogeneity between studies was high (I2 > 40%) and fixed model was appropriate for low heterogeneity between studies (I2 < 40%). For all hypothesis tests, evidence has based on the p - value < 0.05, and the 95% CIs were therefore presented. Weight has indicated the studies value in analysis which depends on confidence intervals and estimated relative risk in Cochran-Mantel-Haenszel method. In the other words, if relative risk has estimated in a confidence interval with high range, the study value and study impact in analysis would be low. Weight has calculated by different software in fixed and random models.
3. Results
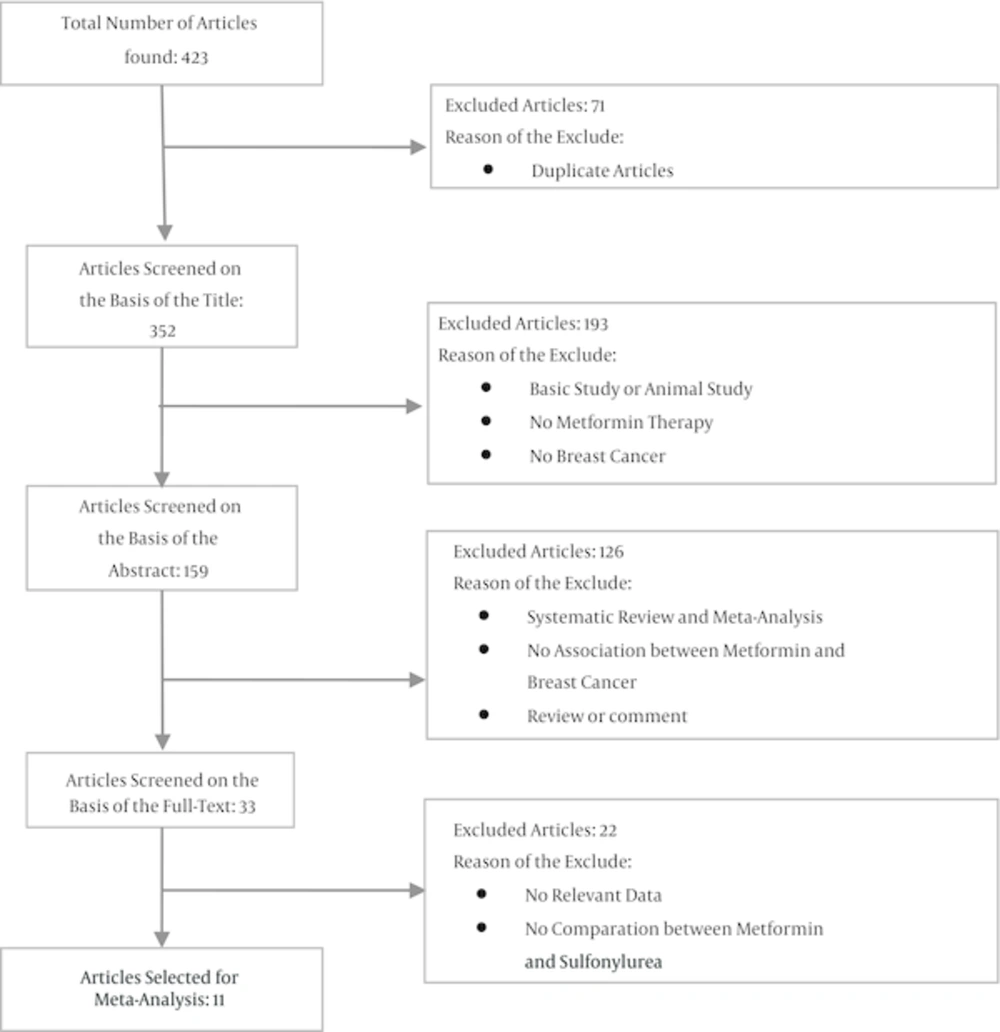
The initial search yielded 423 articles, of which 71 duplicated ones have deleted. From the 352 remaining papers, 319 ones have excluded based on the title and abstract. Studying the full report of the 33 remaining papers has led to the inclusion of 11 papers (Figure 1).
The total number of women in metformin group and Sulfonylurea group were 151646 and 83153 respectively. The mean age of patients was between 35 to 90 years and follow up time after treatment varied between 1 to 12 years. Overall out of these 11 articles, we have included 1 case-control study, 9 cohort studies and 1 RCT study. All the including 11 studies have published between 2009 and 2015. Characteristics of included papers have shown in Table 2.
| First Auhtor | Year | Country | Study Design | Population | Treatment; No. of Patients | Outcome | Follow Up | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Metformin | Sulfonylurea | Total | ||||||||
| Philip D Home | 2009 | UK - Denmark - Spain - Germany - France | RCT | Women with type 2 diabetes (mean age 57.2 ± 10.7 years) | 554 | 557 | 1111 | Breast Cancer Risk | mean 5.5 years | (18) |
| Craig J. Currie | 2009 | UK | Cohort | Women with type 2 diabetes (mean age 62 ± 14.6 years) | 15,364 | 3,354 | 18718 | Breast Cancer Risk | mean 2.4 years | (19) |
| Michael Bodmer | 2010 | Switzerland | Case-Control | Women with type 2 diabetes(mean age 67.5 ± 10.5 years) | 260 | 314 | 574 | Breast Cancer Risk | NR | (11) |
| Ming-Chia Hsieh | 2012 | Taiwan | Cohort | Women with type 2 (mean age 61.44 ± 13.23 years) | 2,048 | 2,804 | 4852 | Breast Cancer Risk | 8 years | (20) |
| Maria Theresa M. Redaniel | 2012 | UK | Cohort | Women with type 2 diabetes (at least 35 years) | 11,918 | 4,815 | 16733 | Breast Cancer Risk | > 5 years | (21) |
| T. P. van Staa | 2012 | UK - Netherlands | Cohort | Women with type 2 diabetes (mean age 63 ± 14 years) | 47,913 | 29837 | 77750 | Breast Cancer Risk | 9 years | (22) |
| Rikje Ruiter | 2012 | Netherlands | Cohort | Women with type 2 diabetes (mean age 61.8 ± 17.6 years) | 28,266 | 16892 | 45158 | Breast Cancer Risk | 3 - 4 years | (23) |
| Hong Qiu | 2013 | USA | Cohort | Women with type 2 diabetes (mean age 60.5 ± 14.9 years) | 16,817 | 6,911 | 23728 | Breast Cancer Risk | mean 5.02 years | (24) |
| Diana Soffer | 2014 | USA | Cohort | Women with type 2 diabetes (mean age 56years) | 4,887 | 8,253 | 13140 | Breast Cancer Risk | 12 years | (25) |
| Konstantinos K. Tsilidis | 2014 | UK | Cohort | Women with type 2 diabetes (mean age 61.1 - 65.3 years) | 22,591 | 7,686 | 30277 | Breast Cancer Risk | Median 5.1 years | (26) |
| Yu-Ching Chen | 2015 | Taiwan | Cohort | Women with type 2 diabetes (Median age 60.6 - 62.4 years) | 1,028 | 1,730 | 2758 | Breast Cancer Risk | Median 2.5 years | (27) |
Characteristics of the Included Articles
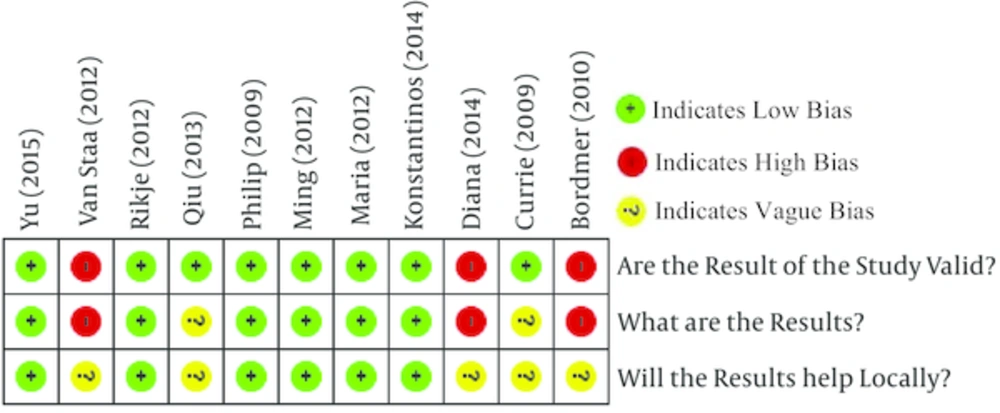
Quality assessment of the articles -which has done with CASP checklist - has shown that out of 11 articles 6 had an acceptable quality, 2 had moderate quality and 3 had low quality (Figure 2).
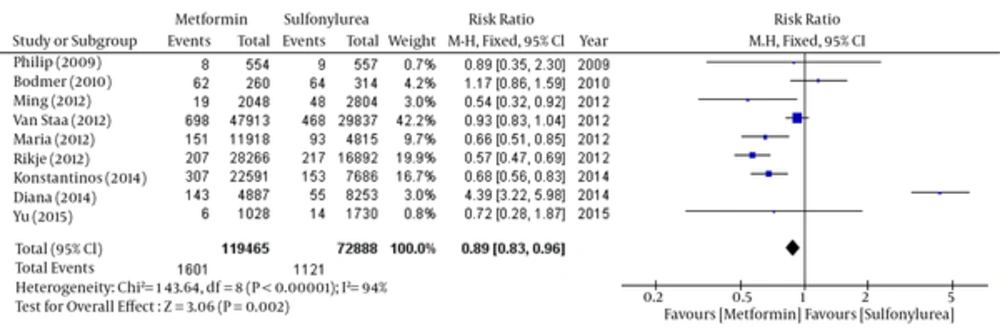
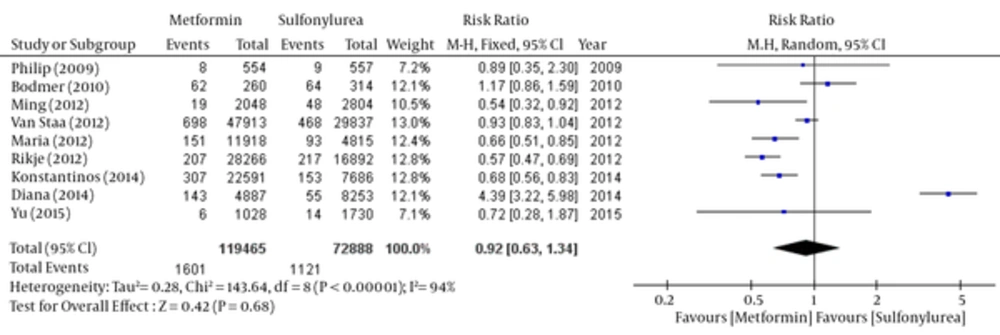
About 9 of 11 papers reported data have associated with breast cancer risk in each group. So we have been able to do meta-analysis the outcome of these 9 articles by Rev-Man software. But because of the lack of some raw data, two other articles had not the ability to be pooled. So we have separated these two articles and done meta-analysis using CMA software. The results of the meta-analysis of nine articles have shown in Figures 3 - 6 and two other papers in Figure 7. Total populations were 119465 in metformin group and 72888 people in the sulfonylurea group in these 9 articles.
The relative risk of breast cancer was statistically significant in favor of Metformin by fixed model (Table 3 and Figure 3), but there was no significant difference between the two groups by using random model (Table 4 and Figure 4).
Fixed: RR: 0.89, 95% CI (0.83 - 0.96), Pv = 0.002.
Random: RR: 0.92, 95% CI (0.63 - 1.34), Pv = 0.68.
| Study | Weight, % | 95% CI | Risk Ratio (Fix Effect) |
|---|---|---|---|
| Philip D Home (2009) | 0.7 | 0.35 - 2.3 | 0.89 |
| Michael Bodmer (2010) | 4.2 | 0.86 - 1.59 | 1.17 |
| Ming-Chia Hsieh (2012) | 3 | 0.32 - 0.92 | 0.54 |
| Maria Theresa M. Redaniel (2012) | 9.7 | 0.51 - 0.85 | 0.66 |
| T. P. van Staa (2012) | 42.2 | 0.83 - 1.04 | 0.93 |
| Rikje Ruiter (2012) | 19.9 | 0.47 - 0.69 | 0.57 |
| Soffer Diana (2014) | 3 | 3.22 - 5.98 | 4.39 |
| Konstantinos K. Tsilidis (2014) | 16.7 | 0.56 - 0.83 | 0.68 |
| Yu-Ching Chen (2015) | 0.8 | 0.28 - 1.87 | 0.72 |
| pooled | 100 | 0.83 - 096 | 0.89 |
Comparison of Breast Cancer Risk between Metformin and Sulfonylurea group; Fixed- Effects Model
| Study | Weight, % | 95% CI | Risk Ratio (Random Effect) |
|---|---|---|---|
| Philip D Home (2009) | 7.2 | 0.35 - 2.3 | 0.89 |
| Michael Bodmer (2010) | 12.1 | 0.86 - 1.59 | 1.17 |
| Ming-Chia Hsieh (2012) | 10 | 0.32 - 0.92 | .54 |
| Maria Theresa M. Redaniel (2012) | 12.4 | 0.51 - 0.85 | 0.66 |
| T. P. van Staa (2012) | 13.0 | 0.83 - 1.04 | 0.93 |
| Rikje Ruiter (2012) | 12.8 | 0.47 - 0.69 | 0.57 |
| Soffer Diana (2014) | 12.1 | 3.22 - 5.98 | 4.39 |
| Konstantinos K. Tsilidis (2014) | 12.8 | 0.56 - 0.83 | 0.68 |
| Yu-Ching Chen (2015) | 7.1 | 0.28 - 1.87 | 0.72 |
| pooled | 100 | 0.63 - 1.34 | 0.92 |
Comparison of Breast Cancer Risk between Metformin and Sulfonylurea Group; Random- Effects Model
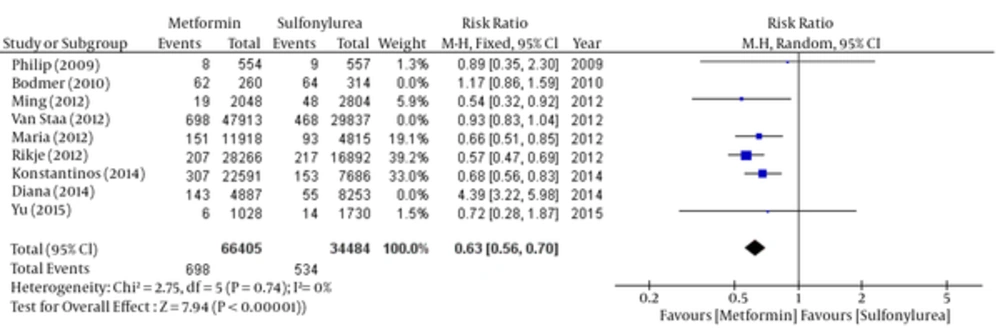
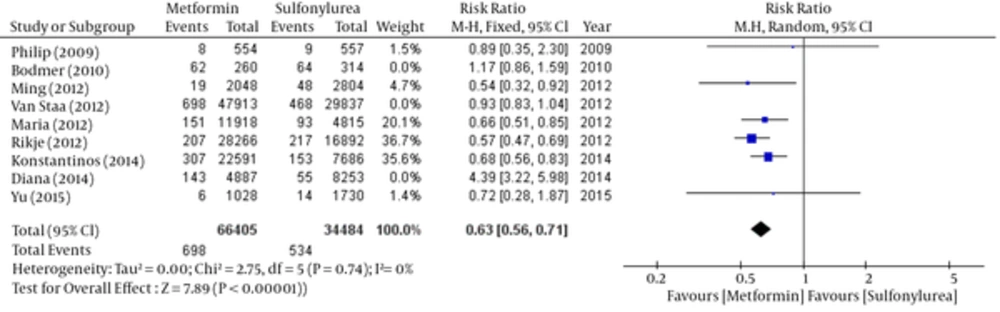
According to the significance of heterogeneity (I2 = 94%) in these studies, both fixed and random model have scrutinized by the step by step removing the studies to investigate the reason of heterogeneity. Finally, by removing three studies (Bodmer, 2010; Van Staal, 2012 and Diana 2014) heterogeneity have eliminated (I2 = 0%). So the relative risk of breast cancer was significant in favor of metformin (Tables 5 - 6 and Figures 5 - 6).
Fixed: RR: 0.63, 95% CI (0.56 - 0.70), Pv < 0.00001.
Random: RR: 0.63, 95% CI (0.56 - 0.71), Pv < 0.00001.
| Study | Weight, % | 95% CI | Risk Ratio (Fix Effect) |
|---|---|---|---|
| Philip D Home (2009) | 1.3 | 0.35 - 2.3 | 0.89 |
| Ming-Chia Hsieh (2012) | 5.9 | 0.32 -0.92 | .54 |
| Maria Theresa M. Redaniel (2012) | 19.1 | 0.51 - 0.85 | 0.66 |
| Rikje Ruiter (2012) | 39.2 | 0.47 - 0.69 | 0.57 |
| Konstantinos K. Tsilidis (2014) | 33 | 0.56 - 0.83 | 0.68 |
| Yu-Ching Chen (2015) | 1.5 | 0.28 - 1.87 | 0.72 |
| Pooled | 100 | 0.56 - 0.7 | 0.63 |
Comparison of Breast Cancer Risk Between Metformin and Sulfonylurea group; Fixed- Effects Model - After Heterogeneity Testing
| Study | Weight | 95% CI | Risk Ratio (Random Effect) |
|---|---|---|---|
| Philip D Home (2009) | 1.5% | 0.35 - 2.3 | 0.89 |
| Ming-Chia Hsieh (2012) | 4.7% | 0.32 -0.92 | 0.54 |
| Maria Theresa M. Redaniel (2012) | 20.1% | 0.51 - 0.85 | 0.66 |
| Rikje Ruiter (2012) | 36.7% | 0.47 - 0.69 | 0.57 |
| Konstantinos K. Tsilidis (2014) | 35.6% | 0.56 - 0.83 | 0.68 |
| Yu-Ching Chen (2015) | 1.4% | 0.28 - 1.87 | 0.72 |
| Pooled | 100% | 0.56 - 0.71 | 0.63 |
Comparison of Breast Cancer Risk Between Metformin and Sulfonylurea Group; Random- Effects Model - after Heterogeneity Testing
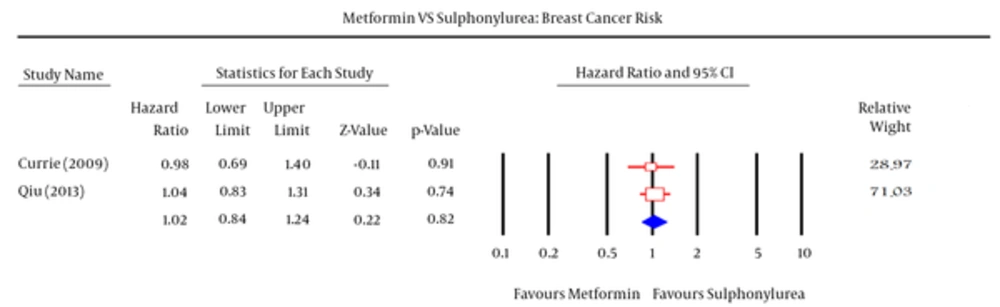
Meta-analysis have done separately in two other studies (Table 7 and Figure 7). There were not a significant differences and significant heterogeneity between two groups in fax and random models (df = 1, I2 = 0, P-value = 0.78).
HR: 1.02, 95% CI (0.84 - 1.24), Pv = 0.82.
| Study | Weight, % | 95% CI | Hazard Ratio |
|---|---|---|---|
| Craig J. Currie (2009) | 28.97 | 0.69 - 1.4 | 0.98 |
| Hong Qiu (2013) | 71.03 | 0.83 - 1.31 | 1.04 |
| Pooled | 100 | 0.84 -1.24 | 1.02 |
Comparison of Breast Cancer Risk Between Metformin and Sulfonylurea
4. Conclusions
Most of research with different methodologies has evaluated metformin in the field of cancer. But the results were inconsistent. The aim of current study was to evaluate the efficacy of metformin compared to sulfonylureas - the most common medicines used in patients with diabetes - in incidence of breast cancer in women with type 2diabetes.
In this study, eleven articles have analyzed that had been published during 2009 to 2015. Among them four articles has conducted in Asia and America and seven articles in Europe. Raw data associated with breast cancer risk in each group have reported in nine articles. So we have been able to do meta-analysis the results of nine articles by Rev-Man software. But the two other articles have been not pooled with nine other articles because of underreporting required raw data. So we have separated these two articles. Meta-analysis has done by CMA software. Because of the heterogeneity between nine articles in Rev-Man software, results have demonstrated low validity about the relative risk of breast cancer. So we have investigated this heterogeneity with step-by-step removing. Finally we have eliminated the disparity between articles for meta-analysis by removing three articles (Bodmer, 2010; Van Staal, 2012 and Diana 2014). In Bodmer study, different design (case-control analysis and retrospective study) and the low levels of evidence were the reason of heterogeneity. In two others study, long-term follow up (9 and 12 years) has led to creation of heterogeneity.
Qiu (24) and Currie (19) studies has indicated that there is no significant difference between metformin and sulfonylureas in incidence of breast cancer by using CMA meta-analysis software. Also there were no statistically significant differences in the results about heterogeneity. Generally, six of eleven articles have indicated that there is no statistically significant difference between two groups. Four articles have indicated significant breast cancer incidence in favor of metformin. On the contrary, one article has indicated significant breast cancer incidence in favor of sulfonylurea. So we have excluded this study from analysis due to the low level of evidence.
A systematic review study (26) was somewhat similar to our study that was conducted in 2012. Its target population was male and female patients with type 2 diabetes and its purpose was metformin and sulfonylurea comparison at incidence risk of any cancer. On the other hand, in this study the numbers of article which exclusively compared metformin with sulfonylurea were only three articles up to 2012. It has seemed that searching for evidences has focused until 2010, While our study has exclusively compared metformin with sulfonylurea in breast cancer. Our target population was only women with type 2 diabetes. And the evidence searching was up to the end of 2015.
Some other previous studies (27-30) have also examined the association between metformin and breast cancer. But the control group was not exclusively sulfonylureas and this was what distinguished our study with these studies. Also what distinguishes our study with Bianca systematic review which has conducted in 2014 were target population and the outcome.
Unfortunately, we have just entered studies that published in English and Persian due to time and resources limitation. But blinding methods has used in selection and quality evaluation stages in order to avoid citation bias.
We have concluded that the incidence of breast cancer in female patients with type 2 diabetes who use metformin significantly was less than those who use sulfonylurea. It has seemed that this difference was due to AMPK activation by metformin which led to reduced insulin levels and inhibition of protein synthesis routes. This process has led to cell growth and proliferation reduction which decreased breast cancer incidence. It should note that these differences in findings have based on observational studies that had low level of evidence. It would be essential that clinical trials should conduct with high sample size.