1. Background
Breast cancer is the most frequent malignancy in women worldwide (1). In Iran, breast cancer is the most common malignancy among women with an estimated age-standardized incidence ratio (ASR) of 28.1 (1). In the United States, around 65% of all women newly diagnosed with breast cancer are > 55 years old, but in most low- and middle-income countries, including Iran, almost half of women with newly diagnosed breast cancer are < 50 years (2). The incidence of breast cancer among Iranian women is increasing. The results of a recent study showed that the rate of mortality from breast cancer in Iran has an increasing trend (3). In order to reduce breast cancer mortality and burden in Iran, preventive and screening programs for breast cancer are needed.
Gene mutations contribute to cancer in 2 ways. Oncogenic mutations that happen in a specific cell after birth are named “somatic cancer mutations” and considered a hallmark of cancer. Genes, in which germline mutations increase the risk of developing cancer, are called cancer predisposition genes. Germline mutations in cancer predisposition genes confer the high or moderate risks of cancer (> 2 fold relative risk) (4). It is estimated that about 3% to 5% of breast cancers are the result of germline mutations in cancer predisposition genes (5). BRCA1 and BRCA2 genes are 2 major high penetrance genes associated with early onset and familial breast and ovarian cancer (6, 7). Women with a germline mutation in BRCA1 or BRCA2 gene have a lifetime risk of breast cancer of up to 70%, and once they are diagnosed with breast cancer, they are at high risk of developing second primary breast and ovarian cancers. Mutation carriers are also at increased risk of prostate, pancreas, and male breast cancer compared to the general population (8-10).
More than 3,000 different genetic variants have been reported in BRCA1 and BRCA2 universal mutation database (11). Despite the large number of carriers detected, only 9 BRCA1 and 6 BRCA2 de novo mutations have been identified (12). It is known that a strong negative fitness effect can result in a high de novo mutation rate (13). Numerous case-control studies between BRCA1 and BRCA2 carriers and non-carriers have shown no effect of these mutations on female fertility (14-16). With respect to the relatively high prevalence of BRCA1 and BRCA2 mutation carriers in the general population (about 1 in 420) and the absence of a negative fitness effect, it seems that most BRCA1 and BRCA2 mutations are inherited and outnumber de novo cases (17). Hereditary breast and ovarian cancer is mainly caused by heterozygous mutation in BRCA1 or BRCA2 genes (18). Biallelic mutations in BRCA2 gene lead to Fanconi Anemia, which is characterized by bone marrow failure and predisposition to cancer (19). In contrast, there is only 1 report of biallelic mutation in BRCA1 gene, identified in a developmentally delayed patient with early-onset ovarian cancer (20).
Several models and scoring systems have been developed to estimate the probability of carrying a BRCA1 or BRCA2 mutation on the basis of personal and family history of breast and/or ovarian cancer (21, 22). The accurate estimation of BRCA1/2 pathogenic mutation likelihood in index cases from families suspected of hereditary breast and ovarian cancer prior to genetic testing is crucial in determining which families should perform costly genetic tests. Manchester scoring system is easier and less time-consuming compared to computer-based models, which makes it more appropriate to use in clinical practice. It consists of 12 components with specific sub-scores for BRCA1 and BRCA2 genes, which are summed up to give a total score. Each component includes the number of breast, ovarian, prostate, and pancreatic cancers diagnosed at different ages in family members of index cases. Several studies have demonstrated the good predictive performance of Manchester scoring system (23-26). A combined score of 16 points is used for the 10% threshold and 20 points for the 20% threshold of BRCA1/2 mutation probability (24).
BRCA1 and BRCA2 mutation frequencies differ considerably among various geographic regions and ethnicities (27-29). Most studies have primarily used Caucasian populations to delineate the population and family risks associated with germline BRCA1 and BRCA2 mutations, leaving patients of other ancestries understudied. As genetic testing for BRCA1 and BRCA2, mutations is underused in Iran, it is of great importance to be able to describe the mutation spectrum of these genes and subsequently the genetic risks and testing benefits particular to Iranian population. Assessment of the literature reporting BRCA1 and BRCA2 mutation frequencies in Iranian population raises concerns about the methodologies and various mutation ascertainment methods used. The identification of mutation carriers leads to the early detection and implementation of strategies to reduce the risk of breast cancer. Furthermore, the management of breast cancer patients who are mutation carriers is different from non-carriers (including bilateral mastectomy and oophorectomy) (30-32). In addition, the results of the TNT trial showed that patients with BRCA1/2 mutations have a greater response and a longer progression-free survival with carboplatin compared with docetaxel (33). Therefore, knowledge of mutation status may have an impact on adjuvant and later treatments, contralateral risk-reduction options, and eligibility for clinical trials. We designed a pilot study to identify the full spectrum of BRCA1 and BRCA2 sequence variations and large single or multi-exonic deletions in a cohort of Iranian breast cancer patients with a high likelihood of hereditary predisposition to breast cancer. Manchester score was calculated for all patients to determine the cut-off value for genetic testing in Iranian families.
2. Methods
Breast and/or ovarian cancer families were identified at Comprehensive Cancer Control Center, Tajrish Shohada Hospital in Tehran, Iran, from September 2014 to April 2017. Twenty index cases diagnosed with invasive breast cancer were selected from 135 unrelated breast and/or ovarian cancer families visited at cancer genetics clinic, with the following inclusion criteria: invasive breast cancer diagnosed ≤ 30 years, bilateral invasive breast cancer and both cancers diagnosed ≤ 40 years, metachronous, bilateral, or ipsilateral invasive breast cancer with ≥ 5 years interval and a first- or second-degree relative with breast cancer diagnosed ≤ 50 years, triple negative breast cancer diagnosed ≤ 40 years, personal history of epithelial ovarian cancer, a first- or second-degree relative with breast cancer and both cancers diagnosed ≤ 40 years, a first- or second-degree relative with epithelial ovarian cancer, male breast cancer with a first- or second-degree relative with breast or ovarian cancer, and a family history with a Manchester score greater than or equal to 16, as previously reported (24).
All study participants provided written informed consent for genetic testing. The study was approved by the ethics committee of Shahid Beheshti University of Medical Sciences. Pre-test genetic counseling was offered to all patients. Genomic DNA was extracted from peripheral whole blood with QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. DNA quantitation was performed with BioPhotometer (Eppendorf, Hamburg, Germany). DNA purity was determined by A260/280 ratio. The entire coding sequence including exon-intron boundaries of BRCA1 and BRCA2 genes was amplified with published primers (34). The primers were synthesized by TAG Copenhagen (Copenhagen, Denmark). The PCR reactions were performed with HotStar Taq Master Mix Kit (QIAGEN, Hilden, Germany) on a Mastercycler (Eppendorf, Hamburg, Germany). The PCR products were sequenced bidirectionally with BigDye Terminator v3.1 Cycle Sequencing Kit on an ABI 3730xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Sequencing traces were analyzed using Chromas 2.6 (Technelysium, Brisbane, Australia). Sequence traces were aligned to BRCA1 RefSeqGene (LRG_292) and BRCA2 RefSeqGene (LRG_293) sequences. Sequence variants were described in accordance with the recommendations of the human genome variation society (HGVS). The DNA sequence numbering is based on cDNA sequences for BRCA1 (NM_007294.3) and BRCA2 (NM_000059.3). Identified sequence variants were interpreted as pathogenic, likely pathogenic, variant of uncertain significance (VUS), likely benign, and benign according to American college of medical genetics and genomics (ACMG) guideline (35).
Large single or multi-exonic deletion testing of BRCA1 and BRCA2 genes with Multiplex Ligation-dependent Probe Amplification (MLPA) method was performed for patients with no identified pathogenic or likely pathogenic sequence variant by Sanger sequencing. MLPA analysis was performed, using probemix P002 BRCA1 and P090 BRCA2 (MRC Holland, Amsterdam, The Netherlands). MLPA results were confirmed by probemix P087 BRCA1 and P077 BRCA2. All MLPA experiments were performed in duplicate, using a Mastercycler (Eppendorf, Hamburg, Germany). Three reference samples (no personal or family history of cancer) were included in each MLPA experiment. MLPA PCR products were separated, using an ABI 3730xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The results were interpreted, using Coffalyser.Net (MRC Holland, Amsterdam, The Netherlands). Post-test genetic counseling was offered to those patients with an identified pathogenic or likely pathogenic sequence variant or large deletion in BRCA1 or BRCA2 genes.
3. Results
The characteristics of 20 unrelated index patients are shown in Table 1. All of the index cases were female, except for patient 13. The mean age at cancer diagnosis of female index patients was 42 years (range 28 - 57 years). Breast cancer was diagnosed in the male index patient at 66 years. Eleven patients were presented with invasive ductal carcinoma, and 2 patients had triple negative breast cancer. Patient 3 had ER/PR negative breast tumor; HER2 immunohistochemistry was 2+, but HER2 in situ hybridization had not been performed. The pathology report of patient 16 was not available. Patients 2 and 17 were not able to present ovarian cancer pathology reports of their first- and second-degree relatives, respectively. Patients 6 and 20 were diagnosed with metachronous bilateral breast cancer with 6 year interval.
| Index Patient (IARC Score) | Age at Breast Ca Diagnosis (Pathology) | Epithelial Ovarian Ca in Index Patient (Pathology) | No. of Female Breast Ca in Family | No. of Epithelial Ovarian Ca in Family (Pathology) | No. of Male Breast Ca in Family | Triple Negative Breast Ca | Bilateral BC | Manchester Score (Pathology-Adjusted) |
|---|---|---|---|---|---|---|---|---|
| 1 (BRCA2+) (5) | 43 (Mixed IDC& ILC) | - | 3 | 0 | 0 | - | - | 20 (21) |
| 2 | 37 (DCIS & Invasive papillary Ca) | - | 1 | 1 (NA) | 0 | - | - | 21 (19) |
| 3 (BRCA1+) (4) | 30 (Invasive medullary Ca) | - | 2 | 1 (Serous cystadenocarcinoma) | 0 | NA | - | 25 (26) |
| 4 | 36 (IDC) | - | 2 | 0 (Dysgerminoma) | 0 | - | - | 10 (6) |
| 5 | 40 (IDC) | - | 2 | 0 | 0 | + (Grade 3) | - | 10 (14) |
| 6 | 43 (Undifferentiated Ca) | - | 1 | 0 | 0 | - | + (6-y interval) | 14 (10) |
| 7 | 41 (IDC) | - | 2 | 0 | 0 | - | - | 14 (10) |
| 8 | 51 (Medullary Ca) | - | 2 | 0 | 0 | + | - | 8 (9) |
| 9 (BRCA1+) (5) | 42 (IDC) | + (Papillary serous cystadenocarcinoma of both ovaries) | 3 | 1 | 0 | + (Grade 3) | - | 31 (35) |
| 10 | 29 (IDC) | - | 3 | 0 | 0 | - | - | 33 (29) |
| 11 | 45 (IDC) | - | 3 | 0 | 0 | - | - | 18 (17) |
| 12 | 57 (IDC) | + (Endometrioid Ca) | 2 | 1 | 0 | - | - | 23 (24) |
| 13 | 66 (IDC) | - | 1 | 0 | 1 | - | - | 18 (17) |
| 14 | 44 (IDC) | - | 2 | 0 | 1 | - | - | 23 (19) |
| 15 | 39 (IDC) | - | 3 | 0 | 0 | - | - | 30 (26) |
| 16 (BRCA1+) (5) | 28 (NA) | - | 3 | 0 | 0 | NA | - | 13 (NA) |
| 17 | 40 (Invasive Tubulo- lobular Ca) | - | 2 | 1 (NA) | 0 | - | - | 25 (24) |
| 18 | 57 (IDC) | - | 3 | 0 | 0 | - | - | 16 (15) |
| 19 (BRCA2+) (5) | 39 (mixed mucinous carcinoma and IDC) | - | 2 | 0 | 2 | - | - | 31 (30) |
| 20 | 53 (Comedocarcinoma) | - | 2 | 0 | 0 | NA | + (6-y interval) | 16 (NA) |
Clinical Characteristics of Index Patients and Their Families
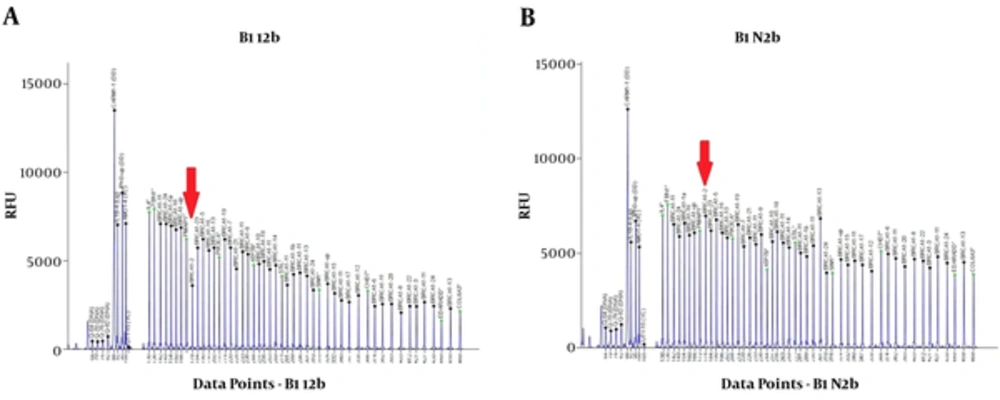
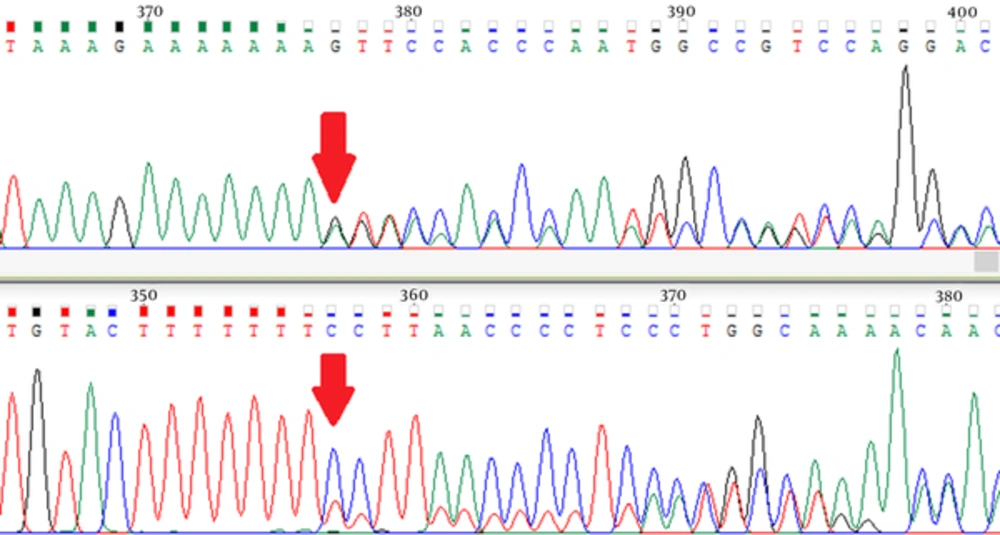
The sequence variants identified in index patients are shown in Tables 2 and 3. Pathogenic or likely pathogenic variants were identified in 5 patients. Patient 1 was carrier of a missense pathogenic mutation in exon 18 of BRCA2 gene: c.8165C > G (p.Thr2722Arg). Patient 3 carried a likely pathogenic mutation in intron 18 of BRCA1 gene: c.5153-26A > G. Patient 9 was carrier of a nonsense pathogenic mutation in exon 15 of BRCA1 gene: c.4566C > G (p.Tyr1522Ter). The MLPA analysis of patient 12 using P002 BRCA1 probemix revealed exon 2 deletion (Figure 1). This patient carried a variant of uncertain significance in exon 2 of BRCA1 gene: c.16C > T (p.Leu6Phe). Further analysis using MLPA probemix P087 did not confirm the deletion of BRCA1 exon 2. Patient 16 was carrier of a frameshift pathogenic mutation in exon 11 of BRCA1 gene: c.1961delA (p.Lys654Serfs*47) (Figure 2). Patient 19 carried a nonsense pathogenic mutation in exon 3 of BRCA2 gene: c.92G > A (p.Trp31Ter). Five variants of uncertain significance (VUS) in BRCA1 gene and 3 variants of uncertain significance in BRCA2 gene were identified.
| Location | Variant | Classification | Zygosity (No. of Patients) |
|---|---|---|---|
| 5ˊ UTR | c.-134T > C | Benign | Heterozygous (11) |
| Exon 2 | c.16C > T (p.Leu6Phe) | Uncertain Significance | Heterozygous (1) |
| Intron 3 | c.134 + 112G > A | Uncertain Significance (VUS) | Heterozygous (2) |
| Intron 7 | c.442-34C > T | Benign | Heterozygous (10) |
| Homozygous (1) | |||
| Intron 8 | c.548-58delT | Benign | Heterozygous (8) |
| Homozygous (1) | |||
| Exon 11 | c.1067A > G (p.Gln356Arg) | Benign | Heterozygous (3) |
| Exon 11 | c.1961delA (p.Lys654Serfs*47) | Pathogenic | Heterozygous (1) |
| Exon 11 | c.2077G > A (p.Asp693Asn) | Benign | Heterozygous (2) |
| Exon 11 | c.2082C > T (p.Ser694 =) | Benign | Heterozygous (10) |
| Homozygous (1) | |||
| Exon 11 | c.2311T > C (p.Leu771 =) | Benign | Heterozygous (10) |
| Homozygous (1) | |||
| Exon 11 | c.2612C > T (p.Pro871Leu) | Benign | Heterozygous (10) |
| Homozygous (2) | |||
| Exon 11 | c.3113A > G (p.Glu1038Gly) | Benign | Heterozygous (10) |
| Homozygous (1) | |||
| Exon 11 | c.3119G > A (p.Ser1040Asn) | Benign | Heterozygous (1) |
| Exon 11 | c.3548A > G (p.Lys1183Arg) | Benign | Heterozygous (10) |
| Homozygous (1) | |||
| Intron 12 | c.4185 + 112C > A | Benign | Heterozygous (1) |
| Exon 13 | c.4308T > C (p.Ser1436 =) | Benign | Heterozygous (10) |
| Homozygous (1) | |||
| Intron 13 | c.4357 + 116G > A | Uncertain Significance (VUS) | Heterozygous (1) |
| Intron 13 | c.4358-77A > C | Uncertain Significance (VUS) | Heterozygous (1) |
| Intron 14 | c.4485-63C > G | Benign | Heterozygous (10) |
| Homozygous (1) | |||
| Exon 15 | c.4566C > G (p.Tyr1522Ter) | Pathogenic | Heterozygous (1) |
| Exon 16 | c.4837A > G (p.Ser1613Gly) | Benign | Heterozygous (10) |
| Homozygous (1) | |||
| Intron 16 | c.4986 + 70G > C | Uncertain Significance (VUS) | Heterozygous (1) |
| Intron 18 | c.5152 + 66G > A | Benign | Heterozygous (10) |
| Homozygous (1) | |||
| Intron 18 | c.5153-26A > G | Likely Pathogenic | Heterozygous (1) |
| Intron 20 | c.5278-191A > T | Benign | Heterozygous (9) |
| Homozygous (1) | |||
| Intron 22 | c.5407-54T > G | Benign | Homozygous (1) |
BRCA1 Gene Variations Detected and Their Clinical Classification
| Location | Variant | Classification | Zygosity (No. of Patients) |
|---|---|---|---|
| 5ˊ UTR | c.-26G > A | Benign | Heterozygous (9) |
| Homozygous (3) | |||
| Exon 2 | c.125A > G (p.Tyr42Cys) | Benign | Heterozygous (1) |
| Intron 2 | c.67 + 82C > G | Likely Benign | Heterozygous (1) |
| Homozygous (1) | |||
| Exon 3 | c.92G > A (p.Trp31Ter) | Pathogenic | Heterozygous (1) |
| Intron 3 | c.316 + 18G > A | Uncertain Significance (VUS) | Heterozygous (1) |
| Intron 8 | c.681 + 56C > T | Benign | Heterozygous (3) |
| Exon 10 | c.1114A > C (p.Asn372His) | Benign | Heterozygous (9) |
| Homozygous (1) | |||
| Exon 11 | c.3396A > G (p.Lys1132 =) | Benign | Heterozygous (12) |
| Homozygous (3) | |||
| Exon 11 | c.3516G > A (p.Ser1172 =) | Benign | Heterozygous (1) |
| Exon 11 | c.3807T > C (p.Val1269 =) | Benign | Heterozygous (9) |
| Exon 11 | c.4563A > G (p.Leu1521 =) | Benign | Homozygous (18) |
| Exon 11 | c.5660C > T (p.Thr1887Met) | Uncertain Significance (VUS) | Heterozygous (1) |
| Exon 11 | c.6131G > C (p.Gly2044Ala) | Uncertain Significance (VUS) | Heterozygous (1) |
| Exon 11 | c.6513G > C (p.Val2171 =) | Benign | Homozygous (20) |
| Intron 11 | c.6841 + 24G > A | Likely Benign | Heterozygous (1) |
| Exon 12 | c.6935A > T (p.Asp2312Val) | Benign | Heterozygous (1) |
| Intron 12 | c.6938-120T > C | Benign | Homozygous (20) |
| Exon 14 | c.7242A > G (p.Ser2414 =) | Benign | Heterozygous (10) |
| Homozygous (2) | |||
| Intron 16 | c.7806-14T > C | Benign | Heterozygous (9) |
| Homozygous (3) | |||
| Exon 18 | c.8165C > G (p.Thr2722Arg) | Pathogenic | Heterozygous (1) |
| Intron 19 | c.8487 + 82G > A | Benign | Heterozygous (1) |
| Intron 21 | c.8754 + 102T > C | Likely Benign | Heterozygous (1) |
| Intron 21 | c.8755-66T > C | Benign | Heterozygous (11) |
| Homozygous (3) | |||
| Intron 24 | c.9257-16T > C | Benign | Heterozygous (2) |
| Exon 27 | c.9976A > T (p.Lys3326Ter) | Benign | Heterozygous (1) |
| Exon 27 | c.10110G > A (p.Arg3370 =) | Likely Benign | Heterozygous (2) |
| 3ˊ UTR | c.*105A > C | Benign | Heterozygous (9) |
BRCA2 Variations Detected and Their Clinical Classification
4. Discussion
Breast cancer imposes a significant health problem in Iran; therefore, dedicated national programs are needed for cancer prevention and early diagnosis. Pathogenic mutations in BRCA1 and BRCA2 genes account for 20% to 25% of familial forms of breast cancer. Professional organizations in Europe and North America have published clinical practice guidelines for BRCA genetic testing and management (36, 37). The utilization of BRCA testing in clinical practice has resulted in a large increase of variant classification burden among laboratories. Variability between genetic code of different individuals is common within the general population and between individuals of different ethnic origin, and this intrinsic variability can lead to difficulties in interpreting some types of sequence change. Variants of uncertain significance (VUS) represent a particular challenge since it is not possible to infer the clinical significance from sequence information alone. Most VUS are not associated with a high risk of cancer, but misinterpretation of VUS can lead to mismanagement of both the patients and their relatives.
In the present study, we performed full sequencing and large deletion analysis of BRCA1 and BRCA2 genes in index patients of 20 high risk Iranian breast cancer families. Two pathogenic and one likely pathogenic variants in BRCA1 gene and 2 pathogenic variants in BRCA2 gene were identified. The MLPA analysis of patient 12 using P002 BRCA1 probemix showed an exon 2 deletion, but subsequent analysis with P087 BRCA1 probemix did not confirm this finding. This patient carried a VUS in exon 2 of BRCA1 gene: c.16C > T (p.Leu6Phe). It is possible that this sequence variant prevented the attachment of P002 exon 2 probe, but not P087 exon 2 probe. Therefore, it is necessary to always confirm MLPA findings with another probemix. A frameshift mutation in exon 11 of BRCA1 gene was identified in patient 16: c.1961delA (p.Lys654Serfs*47). She had a personal history of breast cancer at age 28, a paternal aunt with breast cancer at age 70, another paternal aunt with uterine cancer at age 42, and a paternal cousin with uterine cancer at age 39 and breast cancer at age 44. This patient had a deletion of nucleotide adenine at position 1961, which led to a frameshift change in the coded amino acid from Lysine to Serine at position 654 and a subsequent premature termination of BRCA1 translation 47 codons later (38-40).
A nonsense mutation in exon 15 of BRCA1 gene was identified in patient 9: c.4566C > G (p.Tyr1522Ter). She had a personal history of breast cancer at age 42 and epithelial ovarian cancer at age 43, a sister with breast cancer at age 40, and a paternal aunt with breast cancer at age 42. This patient carried a C > G substitution at position 4566, which led to the premature termination of BRCA1 translation (38, 39, 41). A splicing mutation in intron 18 of BRCA1 gene was identified in patient 3: c.5153-26A > G. She had a personal history of breast cancer at age 30, and her mother was diagnosed with breast cancer at age 58 and epithelial ovarian cancer at age 59. This patient carried an A > G substitution in intron 18 of BRCA1 gene. The analysis of this variant with Human Splicing Finder version 3.0 identified a cryptic new acceptor site at position -37 (42). MutationTaster software identified this variant as disease causing (43). Allele G has neither been found in ExAC (Exome Aggregation Consortium) nor in 1000 Genome projects. COVAR project (COsegregation of VARiants in the BRCA1/2 Gene) by Institute Curie has classified this variant as likely pathogenic on the basis of its family co-segregation (41).
A nonsense mutation in exon 3 of BRCA2 gene was identified in patient 19: c.92G > A (p.Trp31Ter). She had a personal history of breast cancer at age 39, a paternal uncle with breast cancer at age 46, and another paternal uncle with breast cancer at age 80. One of her paternal cousins was diagnosed with breast cancer at age 35. This patient carried a G > A substitution at position 92, which resulted in premature termination of BRCA2 translation (39, 44). A missense mutation in exon 18 of BRCA2 gene was identified in patient 1: c.8165C > G (p.Thr2722Arg). She had a personal history of breast cancer at age 39, a sister with breast cancer at age 41, and her mother was diagnosed with breast cancer at age 45. She also had a maternal aunt with uterine cancer at age 53. This patient carried a C > G substitution at position 8165, leading to substitution of amino acid threonine at position 2722 by arginine. This amino acid is located in the DNA binding domain (DBD) of BRCA2 protein and is considered a pathogenic variant (44, 45).
Assuming a Manchester score of 16 points as cut-off value (10% cut-off) to perform BRCA genetic testing, this scoring system has a sensitivity of 80%, specificity of 33%, positive predictive value (PPV) of 29%, and negative predictive value (NPV) of 83%. On the other hand, when the cut-off value of 20 points (20% cut-off) is chosen, this scoring system has a sensitivity of 80%, specificity of 60%, PPV of 40%, and NPV of 90%. This is similar to the findings of other published studies. Antoniou et al., by analysis of 2140 families in the UK, showed that at 10% cut-off, Manchester score had a sensitivity of 92.3%, specificity of 33.4%, PPV of 24.4%, and NPV of 94.9%. But, when 20% cut-off was chosen, Manchester score had a sensitivity of 87.1%, specificity of 43.4%, PPV of 26.4%, and NPV of 93.6% (26). Similarly, Kast et al. by analysis of 9,390 families in Germany, showed that sensitivity of Manchester score at 10% mutation probability cut-off was 92.2% and specificity was 25.4% (23).
In conclusion, in the present study, we identified 5 different BRCA1 and BRCA2 pathogenic or likely pathogenic mutations in 20 index patients from high risk Iranian breast cancer families. Considering the high cost of BRCA testing in Iran, it seems that Manchester score cut-off value of 20 points is more appropriate in Iranian population. As this scoring system is not capable of identifying all BRCA mutation carriers, a complementary set of criteria similar to the current study and recommendations of other organizations is needed.