1. Background
Ureteral obstruction is one of the complications of prostate cancer. Before the advent of prostate specific antigen (PSA), ureteral obstruction was a common presentation for prostate cancer (1). By the beneficial effect of PSA in early detection of prostate cancer, the initial presentation of this cancer has changed to organ confined disease during the past decades, and locally advanced disease has been limited to only 3% of this population (1, 2). Ureteral obstruction is developed in 3% to 16 % of patients with locally advanced prostate cancer (3), Whereas prostate cancer is the most common solid organ cancer in men (4); ureteral obstruction treatment is one of the main steps in the management of advanced prostate cancer.
Ureteral obstruction can occur because of the direct compression of prostate tumor and extrinsic compression of the lymph node metastasis in retroperitoneal space (1, 3, 5). Obstructive uropathy is a slow process and, ultimately, leads to electrolyte imbalance, urosepsis, renal insufficiency, and death (5, 6).
The optimal management of these kinds of ureteral obstruction remains unclear and it is considered a dilemma in this topic (3, 5-7). Retrograde ureteral stent placement and percutaneous nephrostomy (PCN) are routinely used to relieve obstruction. Both PCN and ureteral stent may reduce quality of life (5, 7, 8). On the other hand, since ureteral stent placement is impossible in the complete ureteral obstruction, urinary diversion remains as the only option.
Many specialists refuse surgical options of ureteral obstruction because of the poor condition and reduced survival of patients with advanced cancer (1, 5, 7, 9), and the majority of studies have focused on other options, such as ureteral stents and PCN. Despite this traditional impression, there is no documented evidence of surgical management rejection in complete ureteral obstruction. In a recent comprehensive study, the median survival of such patients, after relief of ureteral obstruction, was more than previous studies and more than what many specialists expect (7). For the first time, we performed a prospective randomized clinical study to compare the safety and efficacy of ureteral reimplantation versus percutaneous nephrostomy and the impact of these procedures on survival and quality of life.
2. Methods
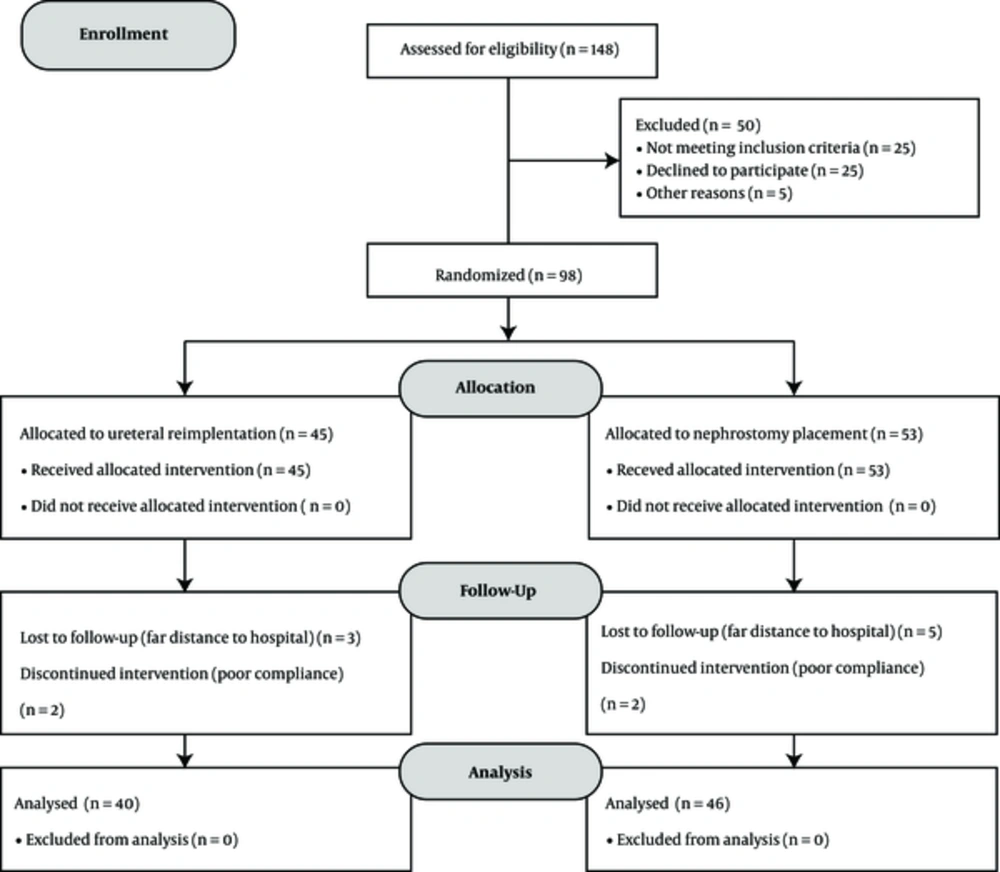
Between January 2010 and March 2014, in a prospective study at the urology department of Shohada-e- Tajrish Hospital, Tehran, 148 patients with hydroureter and hydroureteronephrosis due to locally advanced and metastatic prostate cancer were enrolled. We randomized the patients who met inclusion criteria into 2 groups by simple randomization. Group 1 included the patients who underwent ureteral reimplantation and group 2 included the patients for whom nephrostomy tube was inserted (Figure 1).
All patients suffered from locally advanced or metastatic prostate cancer. Our indications for intervention were increased creatinine level, accompanied with bilateral hydroureter or hydroureteronephrosis or in solitary kidney obstruction. Febrile urinary tract infection or urosepsis, septic shock, and flank pain were the other indications of intervention for hydroureter or hydroureteronephrosis. All patients who participated in this study underwent full medical assessment by anesthesiologic service and all of them had to have anaesthesiology approval for surgical operations. We tried to place retrograde ureteral stent in patients who needed intervention. Patients with successful stenting were excluded from the study. Other exclusion criteria of the current study were history of pelvic surgery, abdominopelvic radiotherapy, and coagulopathy. Basic information of patients, including age, body mass index, and pathology were recorded. Creatinine level, hemoglobin, hematocrit, platelet count, coagulative tests (PT, PTT, and INR), urine analysis, and urine culture were recorded before any intervention. Urinary infection was treated before any intervention if presented.
In group 1, channel TUR would have been performed if the patients had obstructive voiding problems. Creatinine level was recorded in the first day, third day, first week, and first month after operations in both groups. All patients were visited every 3 months after operations, and during the visits, physical examination, creatinine level measurement, and ultrasound examination of hydronephrosis were performed. The mean duration of follow up was 23.87 ± 2.01 months.
In group 1, problems of ureteral anastomosis (leakage and stricture) and wound problems (dehiscence and infection) were assessed during hospital admission, and in 3 months visits, by physical examination, drain output measurement, and ultrasound (if it was necessary).
In group 2, problems of nephrostomy tubes (leakage, kinking, infection, etc) were recorded, according to patients complains and physical examination, biochemical evaluation, and ultrasound examination.
2.1. Nephrostomy Technique
For the placement of nephrostomy tube, patients were fixed in the prone or lateral position. After Prep and Drep, local anesthesia was performed under the supervision of anesthesiologists. Patients were monitored closely by the anesthesiology setting. The optimal location of nephrostomy was assigned by ultrasound. Small incisions were performed in skin and fascia by Bistoury. Afterwards, access was performed by Shiba needle, 18 Guage, under the guidance of ultrasonography. The actual location of the needle was assessed through ultrasound and urine output. Then, J-shape guidewire (0.035 inch) was passed through the needle. By plastic dilatators, the tract was dilated, and a 12 Fr, pigtail nephrostomy tube, was passed over the guide wire. When the location and function of nephrostomy tube were confirmed, it was fixed to skin.
2.2. Ureteral Reimplantation Technique
After performing spinal anesthesia, patients were placed in the supine position. Prep and Drep were done. A 14 Fr Foley catheter was inserted; a 5 cm Para-midline incision was made on skin, the upper point of incision was in the umbilicus level. From an extra peritoneal plan, distal of ipsilateral ureter was delivered from its anatomical position, adjacent to iliac vessels. The ureter was ligated and cut from proximal point to the obstruction level as distal as possible. The dome of the bladder was incised and epithelial to epithelial anastomosis of the ureter to bladder was performed after the placement of short length Double J ureteral stent. Anastomosis was done, using watertight 5 - 0 Vicryl sutures. Other layers of bladder were reconstructed. Hemovac drain was fixed. Foley catheter was removed 3 days after operation. Drain was removed when its output was lower than 25 mm. One month after operation, Double J catheter was removed by cystoscopy under the local anesthesia.
2.3. Ethics
All stages of this study have been supervised by the medical ethics committee of Shohada-e-Tajrish hospital and faculty of medicine of Shahid Beheshti University of Medical Sciences. All patients were aware from the study information and signed informed consent.
2.4. Statistics
All data were described by mean and variance values. The Independent t test was used to compare them. Kaplan-Meier method and Breslow’s test were used for measurements related to survival. SPSS 18 was used for data analysis. A P < 0.05 was considered statistical significant.
3. Results
A total of 98, out of 148, patients met the inclusion criteria in this randomized clinical trial. These 86 patients were randomized into 2 groups: group 1 included 40 patients and planned for ureteral reimplantation, and group 2 included 46 patients who underwent nephrostomy placement. The mean age of patients was 72.20 ± 7.77 years in group 1 and 74.26 ± 7.56 years in group 2, respectively (P = 0.21). Mean Gleason score were 8.02 ± 0.69 in group 1 and 8.00 ± 0.66 in group 2 (P = 0.88). Data related to mean creatinine level before and after interventions, mean PSA, and BMI of both groups were listed in Table 1.
| Group 1 (n = 40) | Group 2 (n = 46) | P Value | |
|---|---|---|---|
| Mean Age, y | 72.20 ± 7.77 | 74.26 ± 7.57 | 0.21 |
| Mean BMI, Kg/m2 | 26.52 ± 5.09 | 26.80 ± 4.86 | 0.79 |
| Mean PSA, ng /mL | 23.10 ± 21.59 | 25.33 ± 23.92 | 0.65 |
| Mean Gleason score | 8.02 ± 0.69 | 8.00 ± 0.66 | 0.86 |
| Mean Cr before intervention, mg/dL | 6.25 ± 2.61 | 6.50 ± 2.81 | 0.66 |
| Mean Cr after intervention, mg/dL | 2.09 ± 0.93 | 2.07 ± 0.87 | 0.91 |
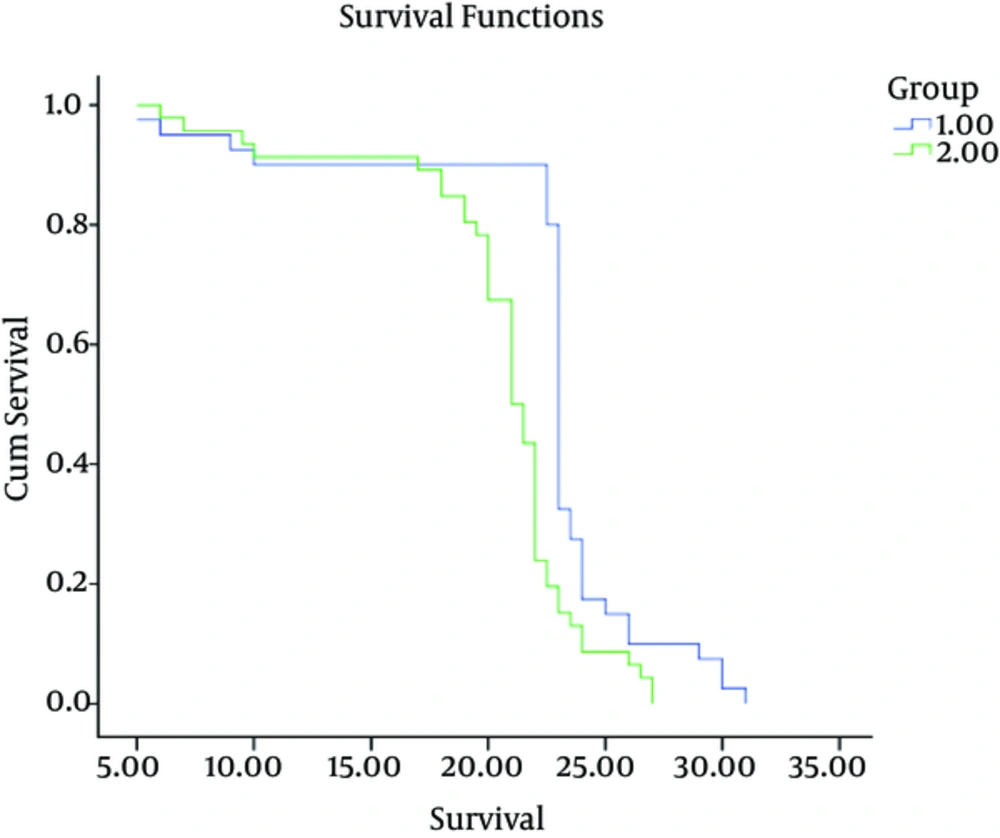
| Mean Survival, m | 22.42 ± 0.87 | 20.48 ± 0.65 | 0.0001 |
aValues are expressed as mean ± SD.
The mean decrease in Creatinine level were 4.16 ± 2.58 and 4.43 ± 2.79 in group 1 and group 2, respectively. The mean decreases in the Creatinine values were not significant between 2 groups (P = 0.639).
All patients were followed for mortality. The mean survival of patients after intervention were 22.42 ± 0.87 months in group1 and 20.48 ± 0.65 in group 2 (P = 0.0001). The Survival rate of patients was significantly higher in group 1 (Figure 2).
Complications in group1 happened in early and late period of time after operation; no major complications happened. Early complications were wound infection 15% (n = 6), fever 17.5% (n = 7), UTI 30% (n = 12), delayed resolved of drain output 12.5% (n = 5), minor hemorrhage 27.5% (n = 11), and seroma formation 5% (n = 2). The mean hospital stay was 96.7 ± 26.2 hours.
The main delayed complications in group 1 were ureteral stricture at the anastomosis point 20% (n = 8), UTI 27.5% (n = 11), and constant pain at the operation site 15% (n = 6). Anastomosis strictures were managed by percutaneous nephrostomy placement.
All patients (100% of them) in group 2 had experienced complications. Complications included febrile UTI 65.2% (n = 30), perirenal abscess 10.8% (n = 5), dislodgement of nephrostomy tube 30.4% (n = 14), local inflammation and dermatitis of nephrostomy tract 54.3% (n = 25), and hemorrhage during nephrostomy placement 4.3 (n = 2). Moreover, all of them (100%) had social inconvenience because of urine bag and nephrostomy tube, local pain and discomfort in tract and sutures of tube, urine leakage and odor, and need to regular replacement of tube.
4. Discussion
Ureteral obstruction develops in 3% to 16 % of patients with locally advanced prostate cancer (3). Obstructive uropathy can cause uremia, electrolyte imbalance, infection and urosepsis, and, ultimately, death. There is no agreement on standard modality to manage this problem (3, 5). Surgical ureteral diversion, ureteral reimplantation, open and percutaneous nephrostomy, and placement of ureteral stent were used for the treatment of ureteral obstruction (5-7, 10, 11).
Nowadays, placement of ureteral stent and nephrostomy are the most common and the most preferred method for the treatment of ureteral obstruction in advanced prostate cancer (5, 7, 12, 13). These methods of obstruction relief have been evaluated in many studies during the past years. Each method has its advantages and disadvantages (13-18). Complications associated with nephrostomy are reported in 4% to 26 % of procedures (15, 19, 20). But, in other studies, complication rates exceed for both PCN and ureteral stents (9). Malposition, occlusion, and infection are some complications of PCN, and Hematoma can also occur during nephrostomy insertion (3). For ureteral stents, failure rates were reported 11%, 56%, and 36% in 2 months, 3 months, and long term, respectively (5, 21, 22). Unfortunately, both PCN and ureteral stents decrease the patients’ quality of life (5). Despite general impression, there is no difference in quality of life between patients with PCN and indwelling ureteral stents (23).
In the present study, 100% of the patients in nephrostomy placement group had experienced complications, including febrile UTI 65.2% (n = 30), perirenal abscess 10.8% (n = 5), dislodgement of nephrostomy tube 30.4% (n = 14), local inflammation and dermatitis of nephrostomy tract 54.3% (n = 25), and hemorrhage during nephrostomy placement 4.3 (n = 2). Also, all of them (100%) had social inconvenience because of urine bag and nephrostomy tube, local pain and discomfort in tract and sutures of tube, urine leakage and odor, and need to regular replacement of tube. Similar to our study Ahmad et al. (24) compared Double J ureteral stenting and percutaneous nephrostomy in obstructive uropathy. They evaluated 300 patients undergoing JJ stenting or percutaneous nephrostomy for obstructive uropathy. Post DJ stent, complications like painful trigon irritation, septicemia, haematuria, and stent encrustation were observed in 12.0%, 7.0%, 10.0% and 5.0% of the patients, respectively. On the other hand, post-PCN septicemia, bleeding and tube dislodgment, or blockage were observed in 3.5%, 4.5% and 4.5%, respectively. PCN appears to be the more reliable approach in the setting of advanced malignancy. Ku et al. (21) reported a greater chance of progressive loss of patency after ureteral stenting compared to PCN in which the incidence of failed diversion secondary to obstruction was 11% and 1.3%, respectively. Feng et al. (25) demonstrated the initial success of stent placement in 71% of the patients with pelvic malignancies with late stent failure in 41%, necessitating PCN placement and 100% success rate. Song et al. (26) reported successful management of ureteral obstruction secondary to gynecological malignancies by ureteral stenting in 67% of the patients with greater trend towards PCN progression noted in patients with tumor invasion of the bladder. Based on the results of the present study and other studies, it seems that PNC is a safe and better method of temporary urinary diversion than double J stenting for the management of obstructive uropathy with the lower incidence of complications.
One of the challenging situations in ureteral obstruction due to prostate cancer is the complete obstruction. In this situation, ureteral passage of stent in retrograde and ante grade fashion is impossible. In these patients, the only way for obstruction relief is urinary diversion. Urinary diversion can be performed by placement of nephrostomy. Nephrostomy tube could reduce the uremia, but problems associated with PCN were mentioned in previous paragraph.
Obstruction relief in complete ureteral can be achieved by surgical procedures, such as conduit diversions and open nephrostomy tubes. These modalities are morbid and out of date (1). Another surgical intervention for treatment of complete ureteral obstruction is bypassing the obstruction by Ureteroneocystostomy. This procedure provides permanent solution for complete obstruction, because the patients are free from long term ureteral catheterization. In 1978, Left and King reported a case of bilateral complete obstruction of ureters, who had undergone ureteroneocystostomy. During the follow up period, significant improvement was observed in renal function of that patient. The survival and long term condition of patient was not observed. Because of the physiologic nature of the Ureteroneocystostomy for such patients and relief of them from nephrostomy tubes, they recommended Ureteroneocystostomy for the selected patients of complete ureteral obstruction due to advanced prostate cancer (10). In 1979, Kihl and Bratt reported the results of Ureteroneocystostomy for 21 patients with bilateral ureteral obstruction due to prostate cancer (11). The overall survival of patients was 10.6 months. In 13 patients living more 6 months after operation, average survival time was 20 months. They also recommended that ureteral reimplantation should be considered in ureteral obstruction treatment.
Many specialists refuse surgical treatment for ureteral obstruction because of poor condition and reduced survival of patients with cancer induced ureteral obstruction (1, 5, 9, 18). Ureteral obstruction in many studies indicates the reduced survival (14, 15, 27-29). In various studies, median survival after ureteral obstruction in malignancies is about, 6 to 7 months (9, 30, 31). This reduced survival may be the main reason for many specialists to avoid surgical interventions, such as Ureteroneocystostomy for such patients; however, recent findings may change this traditional view for surgical interventions in such patients. Recently, Spenser et al. showed a median survival of 16.7 months after the obstruction time among the patients who underwent nephrostomy or placement of ureteral stents for relief of obstruction in a study, using the Surveillance, Epidemiology, and End Results (SEER) database from 2958 patients with ureteral obstruction due to prostate cancer (7). Additionally, in another recent study, Gandaglia et al. according to the information of the 3875 patients presented with metastatic prostate cancer between 1991 and 2009 included in the surveillance epidemiology and end results– medicare database, found that median cancer specific survival of these patients with prostate cancer and lymph node and bone metastasis were 61 and 32 months, respectively (32). We have found similar values for the survival of patients after ureteral obstruction. These findings are different from the previous findings related to survival time. New chemical therapeutic agents and better management of prostate cancer and its complications have resulted in much better survival in such patients. Considering these findings, it is reasonable to give up that dogma in treatment of prostate cancer induced ureteral obstruction.
Regarding the issue of quality of life, all patients in nephrostomy group had complications that severely restricted their activity and life. These complications were not associated with ureteral reimplantation; therefore, patients' satisfaction was much higher in those patients.
5. Conclusions
Reimplantation is a safe and effective option in the management of complete ureteral obstruction due to prostate cancer with significant benefits in overall survival and patient satisfaction. Our study has some limitations. We performed ureteral preimplantation for healthier patients who had anesthesiological approval for operation; thus, the results cannot be expanded to all patients with various health performances. Furthermore, we did not have standard questionnaire for comparing quality of life between 2 groups. Larger and multicenter studies are needed to better evaluate our findings.