1. Background
Breast cancer is one of the most common cancers in women (1). Recent studies suggest that the rising trend of breast cancer has turned the condition into one of the most common malignancies among Iranian women with a prevalence of 120 per every 100,000 women. It is therefore essential to investigate the etiology of breast cancer among Iranian women (2, 3). Breast cancer is a hormone-dependent cancer and the interaction between estrogen and progesterone is involved in its development (4, 5). CYP1B1 belongs to the cytochrome P450 superfamily of enzymes that has been reported to have a strong role in initiating malignancy in organs responding to estrogen, such as the breast, as well as in causing hormone-dependent cancers (6). CYP1B1 is overexpressed in breast tumors and other tumor tissues such as the lungs, the skin, the ovaries and the testicles (7-9).
Cytochrome P450 enzymes have an active presence in all living creatures, from Eukaryotes to Eubacteria and Archaebacteria. These enzymes catalyze a variety of reactions and are part of the electron transfer system in the complex network of endoplasmic detoxification (10). They are also responsible for the catalysis of many reactions, including steroid and other lipid reactions. These hemoproteins (10) are a combination of mono-oxygenases composed of about 500 amino acids and one iron group at their active site. These enzymes catalyze the oxidation of potentially hazardous waste substances by dissolving them in water and use iron for the oxidation of some other compounds (11).
Made up of three exons and two introns, CYP1B1 is located at 2p21-22. Open reading framework (ORF) begins with the second exon and encodes an enzyme with 543 amino acids (12-14); that is, only two exons are encoded from this gene (15). The different polymorphisms of this gene have been reported as m1 (CYP1B1*1), m2 (CYP1B1*2), m3 (CYP1B1*3), m4 (CYP1B1*4), m5 (CYP1B1*5), m6 (CYP1B1*6) and m7 (CYP1B1*7) (16-18), which vary widely across different races and populations.
Various genotypes have been reported for the rs1056836 polymorphism in Asian and European populations (19, 20). The different genotypes of this polymorphism have been examined and their frequency reported in countries such as Poland, China, Sweden, the US, India, and Ethiopia and in Caucasian populations. In Asian and African-American countries, Guanine (G) is normally located at the nucleotide 4326 locus of CYP1B1, which encodes Valine in the protein chain (19), whereas in European countries, Cytosine (C) is located at the same locus of the gene, encoding Leucine (Leu) in the protein chain (21). The wild-type nucleotide located at the 4326 locus of CYP1B1 has been reported to take different positions in different populations.
2. Objectives
The present study is the first to examine the CYP1B1 polymorphism Leu432Val (m3) in relation to the risk of breast cancer development in Iranian women.
3. Methods
The present case-control study was conducted on 79 women with breast cancer whose diagnosis was confirmed through medical examinations and clinical tests performed in Shohadaye Tajrish hospital and 79 women who were confirmed to not have special diseases such as diabetes and hypertension and a history of diseases such as breast cancer, particularly among their first degree relatives.
DNA was extracted from the blood samples using the saturated salt method (22) and the extracted DNA concentration was examined in all the samples. Specific primers complementary to the target area and previously used were selected to perform the polymerase chain reaction (23) in the following order:
Forward: CACTGCCAACACCTCTGTCT-3-5;
Reverse: GCAGGCTCATTTGGGTTG-3-5.
The optimal annealing temperature for the primers was determined as 64°C. The thermocycler was scheduled for one cycle of 5 minutes at 95°C, followed by 37 cycles of one minute at 95°C, 30 seconds at 64°C, 40 seconds at 75°C and finally one cycle of 7 minutes at 72°C for completing the synthesis of DNA strands. The PCR products were loaded on the gel and a 294 bp band was seen according to the marker size.
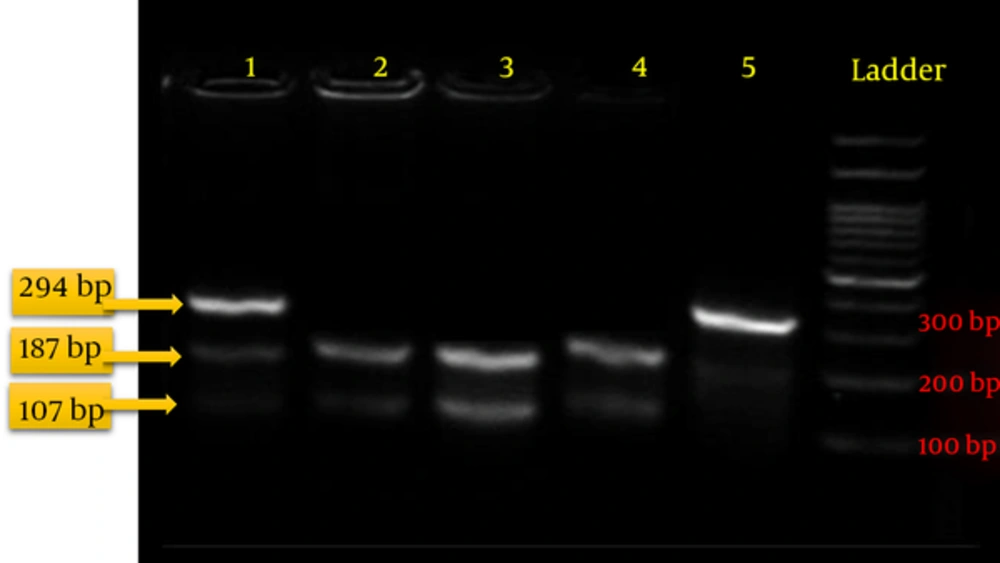
The PCR restriction fragment length polymorphism (PCR-RFLP) method was used for the genotyping. Five units of the Eco571 enzyme were added to the PCR products and the mixture was then incubated at 37°C for 20 - 24 hours. The fragmented pieces were then loaded on agarose 3% for visualization under UV light. Observing three pieces 294 bp, 187 bp and 107 bp in length indicated a GC or heterozygous genotype. Observing two pieces 107 bp and 187 bp in length indicated a CC or mutant homozygous genotype, indicating changes at the target locus. Observing one unchanged piece 294 bp in length indicated a normal homozygous genotype (the absence of polymorphisms at the target locus) (Figure 1).
4. Results
Both groups were at the age range of 32 to 70 years old; the mean age of the participants was 49.68 years old in the cancer group and 47.34 years old in the control group. The mean weight of the participants was 68 ± 8 kg in the cancer group and 61 ± 2 in the control group.
According to their medical records, estrogen receptor expression was observed in 58% and progesterone receptor expression in 42% of the cancer patients.
Among the cancer patients, 26 (32.91%) had Invasive Lobular Carcinoma (ILC), 45 (56.96%) had invasive ductal carcinoma (IDC), 5 (6.33%) had Ductal Carcinoma in Situ (DCIS) and 3 (3.80%) had Lobular Carcinoma in Situ (LCIS). In 38 of the patients, the tumor had metastasized while 16 had grade III tumors, 13 grade II/III and 12 grade II. According to their medical records, estrogen receptor expression was observed in 69.62% and progesterone receptor expression in 41.77% of the cancer patients. Characteristics of patients group are summarized in Table 1.
| Characteristic of Patients Group | No. (%) |
|---|---|
| Invasive lobular carcinoma (ILC) | 26 (32.92) |
| Invasive ductal carcinoma (IDC) | 45 (56.96) |
| Ductal carcinoma in situ (DCIS) | 5 (6.33) |
| Lobular carcinoma in Situ (LCIS) | 3 (3.79) |
| Metastasis | 38 (48.10) |
| Grade III | 16 (20.26) |
| Grade II/III | 13 (16.45) |
| Grade II | 12 (15.19) |
4.1. Data Analysis
The direct counting method was used for finding the frequency of the different alleles and genotypes in the two groups. The data obtained were then analyzed in SPSS-21 using the Chi-square test at a significance level of P < 0.05. The frequency of the alleles was then calculated in both groups using the Hardy-Weinberg equilibrium model and the frequency of the genotypes was then compared between the groups using the Chi-square test.
According to the genotype counting, the homozygous GG genotype was present in 24 (30.38%) of the patients in the cancer group and 26 (32.91%) in the control group (P = 0.732). The CC genotype was present in 25 (31.65%) of the patients in the cancer group and 11 (13.93%) in the control group (P = 0.008). The GC/CG genotype was present in 30 (37.97%) of the patients in the cancer group and 42 (53.16%) in the control group (P = 0.055).
According to the Hardy-Weinberg principle, the frequency of the G allele was 49.37% in the cancer group and 59.49% in the control group. The frequency of the C allele was 50.63% in the cancer group and 40.51% in the control group. According to the results, the C allele had the highest prevalence among the cancer patients with a frequency of 50.63% compared to the G allele with a frequency of 49.37%. The opposite was true for the control group, as the G allele was more prevalent with a frequency of 59.49% compared to the C allele with a frequency of 40.51%, which showed the lowest prevalence in this group.
The odds ratio of the CC genotype was calculated as 2.862 (CI 95%: 1.294 - 6.332 and P = 0.008), showing the significant effect of this genotype in causing the disease. In contrast, the odds ratio of the GG genotype was calculated as 0.89 (CI 95%: 0.455 - 1.740 and P = 0.732), showing that this genotype has no role in causing the disease. Finally, the odds ratio of the CG genotype was calculated as 0.539 (CI 95%: 0.286 - 1.017 and P = 0.055); (Table 2).
| Genotype | Cancer Group | Control Group | P Value | Odds Ratio | CI (95%) |
|---|---|---|---|---|---|
| CC | 25 (31.65) | 11 (13.93) | 0.008 | 2.862 | (6.332 - 1.294) |
| GG | 24 (30.38) | 26 (32.91) | 0.732 | 0.89 | (1.740 - 0.455) |
| GC | 30 (37.97) | 42 (53.16) | 0.055 | 0.539 | (1.017 - 0.286) |
In the cancer group, positive estrogen receptor expression was observed in 16 of the 24 patients with a GG genotype, 21 of the 25 with a CC genotype and 18 of the 30 with a CG/GC genotype. Positive progesterone receptor expression was observed in 13 of the 24 patients with a GG genotype, 11 of the 25 with a CC genotype and 9 of the 30 with a CG/GC genotype.
Data on other factors such as age, weight, disease grade, estrogen receptor (ER) and progesterone receptor (PR) were extracted from the patients’ records so as to analyze the relationship between the genotypes and each of the factors. The results obtained showed that a significant relationship existed only between the CC genotype and disease grade (P = 0.046).
5. Discussion
The prevalence of breast cancer is increasing in many countries; however, mortality rates have remained constant or have insignificantly dropping in some cases. In Iran, the mean age of developing breast cancer is 48.8, with the highest rate of malignancy occurring in the 40 - 49 age group (31.8%) and the lowest in the below-40 age group (23%) (24).
CYP1B1 located in the endoplasmic reticulum plays a major role in activating several environmental carcinogens such as polycyclic aromatic hydrocarbons (PAHs) (25) and aromatic amines (AAs) (26) and in metabolizing 17β estradiol (25). This cytochrome can catalyze 17β estradiol (E2) into catechol metabolites of estrogen, including 2-OH-E2 and 4-OH-E2, which play a major role in breast carcinogenesis. The active metabolites produced by CYP1B1 are able to harm DNA. CYP1B1 may therefore have a significant role in tumorigenesis. The role of CYP1B1 in fetal development has recently been uncovered. The gene of this protein is also expressed in several extrahepatic tissues such as the breast, uterus, kidney, prostate and lungs (26).
Studies suggest that four CYP1B1 polymorphisms at codons, including (CYP1B1*1) Arg48Gly, (CYP1B1*2) Ala119Ser, (CYP1B1*3) Leu432Val and (CYP1B1*4) Asn453Ser, increase hydroxylation activity more than the normal varieties (27, 28). Polymorphisms at codons including (CYP1B1*2) Ala119Ser and (CYP1B1*3) Leu432Val have also been found to increase enzyme activity by two to four times the normal varieties (27). Studies have shown that both the homozygous and heterozygous genotypes of the Leu432Val polymorphism are associated with an increase in the risk of breast cancer to the same amount as ovarian cancer (29-32). Some studies have shown that replacing a G allele with a C allele can be associated with an increased risk of various cancers such as lung (15, 33), prostate (34), breast (20, 35-37) cancer and bone abnormalities (37).
Studies have examined the relationship between CYP1B1 polymorphisms and breast cancer among Chinese, Japanese and Turkish women. The risk of breast cancer increases dramatically in women with CYP1B1 432Val polymorphisms through an exposure to tobacco smoke and a prolonged use of hormones (hormone replacement therapy) (27-30, 37).
Martinez-Ramirez et al. (2013) (38) showed that polymorphisms including CYP1B1 rs1056836, CYP1A1 rs1048943, COMT rs4680, GSTP1 rs1695, GSTT1 null and GSTM1 null in the estrogen metabolic pathway are related to an increased risk of breast cancer in Mexican women. Haiyan Jiao et al. (2010) (39) found a relationship between the polymorphisms in exon 2 of CYP1B1 (codon 119 (G→T)) and exon 3 of this gene (Codon 432, G→C) and an increased risk of breast cancer among the Chinese. Zimarina TC et al. (40) found three polymorphisms of CYP1B1, including Arg48Gly, Val432Leu and Ala119Ser, to be associated with an increased risk of breast cancer and endometriosis; however, De Vivo et al. (2002) (6) found no significant relationships between polymorphisms including Val432Leu (m1) or Ala453Ser (m2) and the risk of breast cancer in Caucasian women. But other studies confirmed the association between rs1056836 and hepatocellular carcinoma risk (41) and laryngeal cancer (42).
The present study found a significant relationship between the rs1056836 polymorphism and breast cancer, but no significant relationships between the cancer group (30.38%) and the control group (32.91%) in terms of the homozygous genotype GG; (P = 0.732). Nevertheless, a significant relationship was found in terms of the CC genotype between the cancer group (31.65%) and the control group (13.93%) (P = 0.008). As for the GC/CG genotype, no significant relationships were observed between the two groups (P = 0.06). The high frequency of the C allele in the cancer group (50.63%) compared to in the control group (40.51%) and the odds ratio of CC genotype shows the significant effect of this genotype in causing the disease. A significant relationship was also found between the CC genotype and the disease grade (P = 0.046).
Given that breast cancer is a complex disease with various factors joining to cause its incidence, no single factor can be said to be solely responsible for its development. Nevertheless, identifying the different factors contributing to its incidence can help improve its prognosis and facilitate its early diagnosis. The results of the present study suggest that CYP1B1 rs1056836 polymorphism may be associated with the susceptibility of breast cancer. Further studies are recommended to be conducted with larger sample sizes in order to assess the accuracy of these findings.