1. Background
Cancer immunotherapy is the aiding of the immune system for treatment of cancers. Two vast strategies are commonly used for cancer immunotherapy: First, the cytotoxic agents that bind immunologically to tumor cells and second, the agents that induce effector immune cells for killing the tumor cells (1). One of the most important fields in modern anti-cancer therapy involves the use of monoclonal antibodies, which selectively and efficiently bind to tumor cells after administration to patients. Various kinds of monoclonal antibodies (mAbs) have been developed and approved for immunotherapy in malignant diseases (2). The mAbs are considered as the most important class of anti-cancer agents, so that around 20 mAbs are in current clinical use, and many are being introduced (3).
Anti-cancer mAbs act through different mechanisms such as direct binding to the malignant cells, changing the host immune response, and delivering cytotoxic substances towards the malignant cells (4). Four kinds of mAbs including murine, chimeric, humanized, and fully human mAbs are in current use for treatment of various malignant (also non-malignant) diseases (5).
Rituximab, a chimeric murine/human anti-CD20 mAb which binds to the B-cell surface marker CD20, is used for the treatment of B cell lymphomas. However, it is used for the treatment of different autoimmune disorders (6, 7). Treatment with Rituximab results in depletion of B-cells from the peripheral circulation of patients (8, 9).
Trastuzumab is a humanized mAb that binds to the extracellular region of HER2 receptor on epithelial cells and inhibits the proliferation and survival of HER2-positive tumors. It was approved by the Food and Drug Administration (FDA) for patients with invasive breast cancer with overexpression of HER2 (10). Bevacizumab (Avastin) is a humanized mAb against the VEGF-A, which is used for the treatment of several vascular cancers such as colorectal cancer, non-squamous, non-small cell lung cancer (NSCLC), glioblastoma, and renal cell carcinoma. This mAb acts through binding to soluble VEGF, preventing its ability to bind to its target receptor, and suppressing the VEGF-related stimulation of pro-angiogenic signaling pathways (3, 11).
Administration of the mAbs directed against tumor-associated antigens may induce development of human antibodies to some parts of administered immunoglobulins such as Fab, Fc, CDR, and idiotype regions (5). Induced human antibodies to mAb inhibit the binding of the administered mAbs to target tumor antigens and also increase the removal of mAbs through reticuloendothelial system in spleen and liver leading to its clearance from blood (12). Moreover, the anti-mAb antibodies may lead to allergic reactions, serum sickness, and renal failure (13, 14). In addition, anti-mAbs may interfere with many types of immunodiagnostic techniques (15), and test results lead to the misdiagnosis and consequently inappropriate treatments (14, 16). Therefore, the detection of anti-mAbs in malignant patients is important to prevent inappropriate decisions. This was the first study in Iran on the evaluation of human antibodies against 3 mAbs i.e. Rituximab, Trastuzumab, and Bevacizumab in cancers including CD20 positive B-Cell malignancies (non-Hodgkin lymphoma and leukemia), breast cancer, and adenocarcinoma (ovarian and colorectal cancer) in patients from Kerman, Iran.
2. Methods
2.1. Subjects
During July 2015 to March 2016, 98 patients (age 53 ± 12.5 years, (range: 26 - 85 years)) with malignancy, who were admitted to Bahonar Hospital (affiliated to the Kerman University of Medical Sciences), Kerman, Iran, were enrolled into the study. The patients were allocated into 3 groups according to the type of administered mAb. Thirty-two patients had lymphoma or leukemia, 43 patients had breast cancer, and 23 patients had colorectal adenocarcinoma.
The patients with lymphoma /leukemia were treated with Rituximab (Reditux®, CinaGen, Iran) in 375 mg/m2 every 3 weeks for 6 months (as initial course) and, then, every 3 months intervals up to 2 years as maintenance course.
The patients with breast cancer were treated with Trastuzumab (Herceptin®, Roche, Germany) at 8 mg/kg as initial dose and 6 mg/kg maintenance dose in 3-week intervals.
The patients with adenocarcinoma were treated with Bevacizumab (Avastin®, Roche, Germany) at 7.5 mg/kg at 3-week intervals.
In all patients, the blood samples were collected 1 to 3 times during immunotherapy program immediately and before the administration of the therapeutic mAbs (Tables 1 and 2). This study was approved by the Ethics Committee of Kerman University of Medical Sciences (IR.KMU.REC.1396.1431). Informed written consent was obtained before the study.
Detection of human antibody against mAbs in patients’ sera was performed by laboratory-developed sandwich ELISA. In this assay, polystyrene high-binding F96 micro plates (Greiner bio one, Germany) were coated overnight at 4°C with 1 µg/mL of any of the administered mAbs (Trastuzumab, Bevacizumabor Rituximab) in 10 mM carbonate/bicarbonate buffer (pH = 9.6). Blocking step was performed by the use of phosphate buffer containing 1% bovine serum albumin (BSA) at room tempreture for 1 hour with constant stirring. Then, 100 µL of serum samples of patients, control samples, and standard samples with known concentrations (in appropriate dilutions with BSA 1%) were added in duplicate to the appropriate wells of micro-plates and incubated at room temperature for 1 hour. The wells were washed 5 times with washing solution containing 0.2% tween. After that, the biotinylated forms of the mAbs were added to the related plates. After 1-hour incubation in ambient temperature, the plates were washed 5 times and incubated for 30 minutes with streptavidin-HRP, washed 5 times, and tapped dry. A fresh TMB/substrate solution (100 µL) was added and the plates were incubated for 20 minutes at dark in ambient temperature. The enzyme reaction was stopped, using 100 µL of 2 N H2SO4 and the optical density for each well was measured by ELISA reader (ELX-608, USA) at 450 nm (and a reference wavelength of 630 nm). The serum concentrations of the human anti-drug antibody against the administrated mAb was determined, using a standard curve that was plotted based on the serial dilution of the rabbit samples with known concentrations of the antibody against the mAbs. The serum concentrations of the human antibody against the used mAb were expressed as AU/mL. The cut off values (mean ± 2 SD AU/mL) were set according to the concentration of anti-mAb levels in the sera of control patients (16 lymphoma patients, 27 breast patients with cancer, and 22 adenocarcinoma patients with standard chemotherapy schedule who did not receive mAbs). The anti-mAb concentrations of 1.76, 0.76, and 0.63 AU/mL were used for discriminating the positive from the negative samples in sera from Rituximab, Trastuzumab, and Bevacizumab-treated patients, respectively. The CV% was 1% and 9% for intra- and inter-assay evaluation. The minimal detection limits were 0.134, 0.057, and 0.137 AU/mL for determination of human anti-mAbs in sera from Rituximab, Trastuzumab, and Bevacizumab-treated patients, respectively.
| Total Number of Infusions | Number of Patients | Seropositive Patient(s) |
|---|---|---|
| 1 - 5 | 14 | 0 |
| 6 - 12 | 11 | 1 |
| 13 - 28 | 7 | 3 |
aThe anti-mAb concentration level of 1.75 AU/mL was used to discriminate positive from negative cases. Accordingly, 12.5% of Rituximab-treated patients were considered as seropositive for antibody against administrated mAb.
| Total Number of Infusions | Number of Patients | Seropositive Patient(s) |
|---|---|---|
| 1 - 3 | 25 | 1 |
| 4 - 7 | 9 | 1 |
| 8 - 13 | 9 | 5 |
aThe anti-mAb concentration level of 0.76 AU/mL was used to discriminate positive from negative cases. Accordingly, 16.3% of Trastuzumab-treated patients were considered as seropositive for antibody against administrated mAb.
2.2. Statistical Analyses
Differences in variables were analyzed, using Spearman and Chi-Square tests as appropriate (P < 0.05).
3. Results
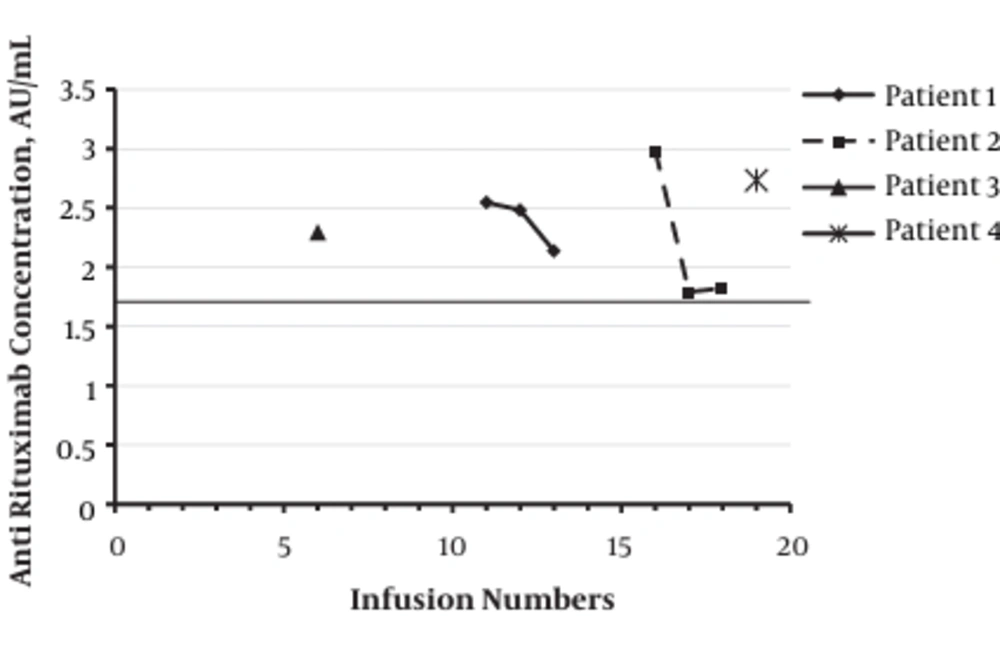
Of the 32 Rituximab-treated patients, 28 (87.5%) patients were negative for the presence of antibody against mAb during initial or maintenance treatment program. Totally, 4 patients (12.5%) were seropositive for the presence of antibody against Rituximab during initial or maintenance treatment program with the mean titer of 2.33 ± 0.37 AU/mL (median: 2.29 (range: 1.79 - 2.98 AU/mL)). Statistical analysis showed no significant association between the number of Rituximab administration and the titer of anti-mAb. The titer of anti-mAb in seropositive patients treated with Rituximab is demonstrated in Figure 1.
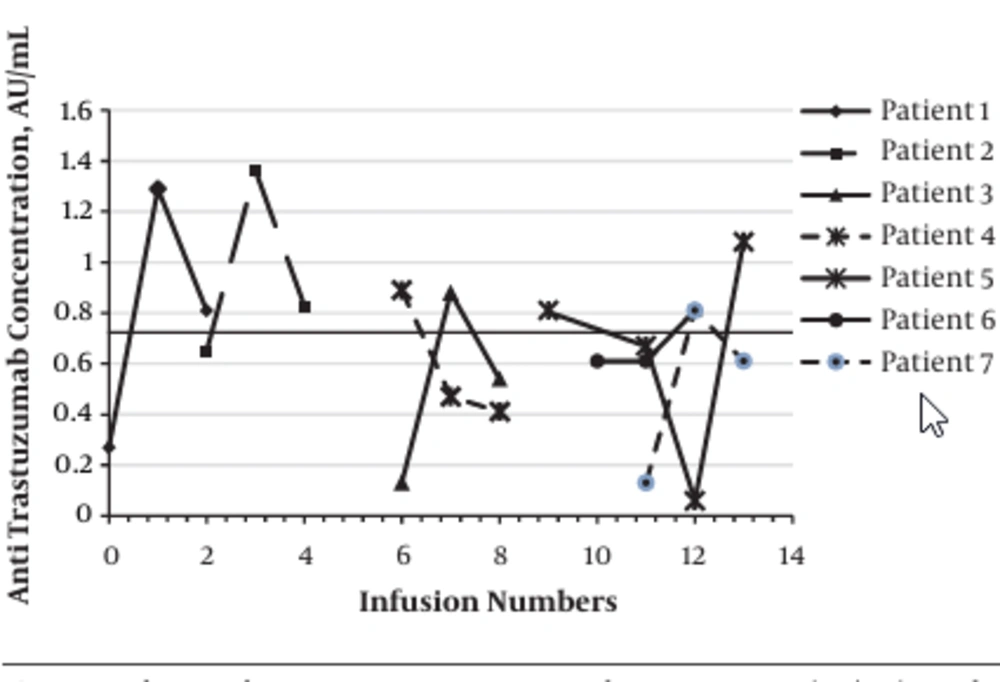
The Rituximab-treated patients were classified into two subgroups according to the receiving of chemotherapy treatment: 15 patients with maintenance course of mAb treatment without chemotherapy and 17 patients in the initial course of treatment with both chemotherapy and immunotherapy. The seropositivity for the presence of anti-mAb in patients treated with Rituximab alone was significantly higher than patients administered both Rituximab and chemotherapeutic agents (26.6% vs. 0.0%; P < 0.02). Of the 43 Trastuzumab-treated patients, 36 (83.7%) patients were negative for the presence of antibody against mAb during initial or maintenance treatment program. Totally, 7 (16.3%) patients were seropositive for the presence of antibody against Trastuzumab during initial or maintenance treatment program with the mean titer of 1.20 ± 0.21 AU/mL (median: 0.85 (range: 0.81 - 1.36 AU/mL)). Statistical analysis showed no significant association between the number of Trastuzumab administration and the titer of anti-mAb. The titer of anti-mAb in seropositive patients treated with Trastuzumab is demonstrated in Figure 2. The seropositivity for the presence of anti-mAb in patients treated with Trastuzumab alone was not significantly different compared to patients administrated both Trastuzumab and chemotherapeutic agents.
None of Bevacizumab-treated patients developed antibody against administrated mAb.
4. Discussion
The results of the present study showed that 12.5% of Rituximab-treated patients and 16.3% of Trastuzumab-treated patients were seropositive for the human antibody against administered mAbs. The development of antibody against mAb was reported in none of Rituximab-treated patients with non-Hodgkin’s lymphoma (17), in 18% of patients with severe pemphigus (18), in 10.6% of patients with rheumatoid arthritis (19), and none of patients with diffuse large B-cell lymphoma (20), who were treated with Rituximab during their therapeutic program.
The results of this study also demonstrated that seropositivity for the presence of anti-mAb in patients treated with Rituximab alone was significantly higher than patients administered both Rituximab and chemotherapeutic agents. These results represent that treatment with chemotherapeutic drugs has negative influences on the development of the anti-monoclonal antibodies.
The development of antibody against mAb was reported in 2.8% of the healthy men, who received a single infusion of Trastuzumab (21) and in 0.5% of women with metastatic breast cancer (22), who were treated with Trastuzumab during their therapeutic program. There is considerable controversy in different studies regarding the development of human antibodies against administered mAb in Rituximab or Trastuzumab-treated patients. These differences may be attributed mainly to the variations in the age, gender, race, genetic background, socioeconomic parameters, other related diseases, design of the study, inclusion and exclusion criteria, doses and timing of the mAb administration, and influences of the other treatment programs.
The development of the human antibodies against mAb in patients with malignant diseases was lower compared to patients with autoimmune disorders. The immune status of patients also may strongly influence the development of human antibodies against mAb. Accordingly, the immunocompromising status in patients with cancer may be responsible for lower production of the human antibody against mAb than patients with autoimmune disorders.
Rituximab is a chimeric mAb, whereas Trastuzumab is a humanized mAb. Therefore, the foreignness degree of Rituximab is more than Trastuzumab. However, we have observed that the seropositivity was lower in Rituximab-treated patients compared to Trastuzumab-treated patients. The diminished counts of B-cells and treatment with hydrocortisone as a potent immunosuppressive agent may be responsible for lower seropositivity in Rituximab-treated patients.
4.1. Conclusions
In conclusion, the results of this study indicate the production of antibody against therapeutic mAbs, Rituximab, and Trastuzumab in a number of treated patients that may influence their efficacy. The production of the human anti-mAb may be influenced by chemotherapeutic drug in Rituximab-treated patients. Bevacizumab did not show immunogenicity in the treated patients.