1. Background
Cancer is a major public health concern all over the world and the major culprit of one of the 5 causes of death in all age groups in males and females (1). There were 14.1 million new cases of cancer and 8.2 million deaths caused by cancer in 2012 globally. The Age standardized rate (ASR) is a summary measure of the rate or weighted average of the age-specific rates based on standard population that is usually world standard population. Age standardized rates can be compared easily between different countries. Age standardized incidence rate of cancer per 100,000 population is 205.4 and 165, respectively, among men and women all over the world. Its age standardized mortality rate is 126.3 and 82.9 per 10,0000 population among men and women, respectively. Incidence rates have more regional variability than mortality rate. There is also more variability among men than women (2).
Generally, 57% of new cancer cases happen in less developed regions (2). For all cancers combined except non-melanoma skin cancer, men and women in the Asian ethnic groups have significantly lower risk of getting cancer than White ethnic groups (3).
The most common cancers identified globally altered slightly over the last 40 years (4-8). ASR for all cancers in Great Britain increased by 23% and 43% in men and women during 1975 to 2009, but over the past 10 years (2000 - 2011), the ASR increased by 3% and 7% in men and women (2). Socioeconomic deprivation is one of the important factors, which affect cancer. ASR is lower in the least deprived groups than the more deprived groups (9). “Westernization” effect is associated with human development index (HDI) and low HDI countries are likely to have increased the incidence of breast, prostate, and colorectal cancers. Based on projections to 2030, if the recent trends in major cancers continue, the burden of cancer will increase 68% compared with 2012, and a higher percentage of this increase will happen in low and medium HDI countries and lower percentage in high and very high HDI countries; Iran is currently among the high HDI countries (2, 10). Cancer is a health concern and the third most common cause of death in Iran (11). Studies have shown that cancer incidence varies across areas (12, 13) and this variations in cancer rates is probably attributed to the environmental risk factors (14, 15), geographical (16-18), and ethnic (19, 20) variations rather than genetic differences.
Removing disparities particularly in cancer incidence is considered as a main health policy. Understanding the disparity status and its causes and scope help us to remove it (21).
Surveillance programs for cancer incidence, its distribution across locations, and its time trend are important for cancer research. Besides, future projection of cancer incidence according to the current trends helps policy makers to make the right decision on establishing programs for control and decrease in the burden of this disease.
The aim of the current study is to demonstrate changing the trends of cancer incidence from 2006 to 2009 in Tehran (capital city of Iran) and evaluate the effect of living in different municipality regions of this city (as a proxy for all contextual factors related to living in that region and as ecological variables have some effect on getting cancer) on the rate of cancer after adjustment for mean age, literacy rate, and employment rate in these regions.
2. Methods
2.1. Study Design
A cross sectional study on the pathologic-based data was designed. We examined all the cancer cases registered in the pathology and hospital base cancer registry of Ministry of Health during a 4-year period of 2006 to 2009; they all were residents in Tehran. This registry collects information on approximately 80% of cancer cases (22, 23). This article was a secondary data analysis on cancer registry data and all the personal data of individuals were confidential. The proposal of the paper was confirmed by Shahid Beheshti University of Medical Sciences research committee and ethical committee.
2.2. Data Sources
Tehran metropolitan area is divided into 22 municipal districts. Population number, employment, and literacy rate of each district in strata defined by each sex and 5-year age groups were obtained from the census data of Iran Statistics Center in 2005. The population number and literacy rate of the following years after census 2005 was estimated by applying the population growth rate for each sex-age group strata across all municipality districts and regions. Employment rate was calculated by dividing the numbers of employed people by population more than 10 years old (this variable is registered in the census for the population over 10 years old) and literacy rate was calculated by dividing the number of literate people by population more than 6 years old in each sex age group strata across regions and municipality districts.
2.3. Statistical Analysis
The age and sex standardized incidence rates and their 95% confidence intervals using the direct method and World Health Organization (WHO) standard population of 2000 were computed.
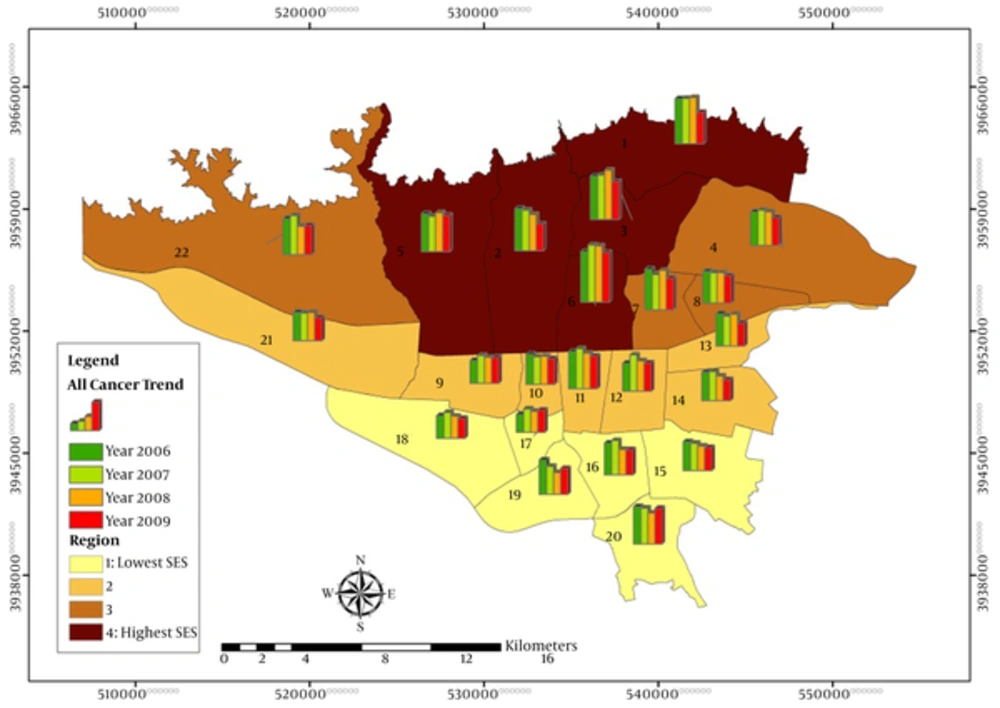
The trend of cancer rate during the 4-year period of 2006 to 2009 was estimated in the Tehran and all its 22 districts. We also grouped Tehran districts into 4 regions, according to their median socioeconomic status (SES), which were defined previously in a study titled “Measuring socioeconomic disparities in cancer incidence in Tehran, 2008” (12). SES index was calculated based on household assets, housing characteristics, and education level. Assets and house features consisted of house ownership, room per person, area per capita, having bath, kitchen, toilet, car, phone, cell phone, freezer, computer, and the years of education were also calculated for individuals older than 6 years across all 22 districts of Tehran. Districts 15 to 20 were categorized in region 1 that located in south of Tehran denoting the lowest socioeconomic status (SES), districts 9 to 14 and 21 located in middle of Tehran categorized in the region 2, districts 4, 7, 8, 22 were classified in region 3 located and districts 1 to 3 and 5 and 6 were categorized in the region 4, which were located in north of Tehran with highest socioeconomic index. The change of cancer trend in these regions and their interactions were also assessed.
Poisson regression and negative binominal regression model were used to assess the significance of incidence trends across 4 consecutive years from 2006 to 2009, as well as the effects of living in regions and municipality district on incidence rate. The above model was also adjusted for the literacy and employment rate of each region and district as contextual factors, which might explain part of the disparity of incidence rate among regions in each sex-age group combination. Since literacy and employment rate were not meaningful in age-group less than 10, this model was fitted on the population of more than 10 years old.
Four models with increasing complexity were fitted to the data. In model zero, the effect of calendar year and age group on incidence rate I each region were estimated. In model 1 and 2, municipality districts and literacy rate were added to the model zero, respectively. In model 3, employment rate was added to the previous model (Models 1 - 3 for districts are available in the supplementary file). Whenever the dispersion in the data was more than a true poison distribution, we changed to a negative binomial regression model. Considering the difference between cancers rates in both sexes, all the models were fitted separately in both sexes. Model selection was according to likelihood ratio test and Akaike’s information criteria (AIC).
Annual percent changes (APCs) for the incidence rates can be calculated as difference between 2 years divided by base year incidence rate times 100. For estimating APCs, we used the following formula; (exp(β) - 1) × 100, where β is the coefficient of variable year in the model adjusted for all above-mentioned variables (24, 25). All the analyses were done by Stata 12.0 software and for mapping, we used ArcGIS 10.
3. Results
3.1. Incidence Trend
During the 4-year period, 30 827 cancer cases were registered in Tehran from 2006 to 2009 with male to female sex ratio of 1.09. The age-standardized incidence rate (ASR) was higher in men compared with women in this period. The sex ratio ranged from 1.08 to 1.15. ASRs for 4 years were 114 and 101 per 100,000 men and women in Tehran, respectively. The highest ASR was 123 among men in 2006. Average annual percent change (AAPC) based on ASR and regression model for each sex was the same and around -5% and -6%, respectively. Table 1 shows crude, ASR and their confidence intervals, and APC. Figure 1 depicts ASR cancer incidence rates in Tehran.
| Sex | Year | Crude Incidence Rate (Per 100,000) | ASR*(CI 95%) Per 100,000 | APC** |
|---|---|---|---|---|
| Men | 2006 | 99.9 | 122.9 (119 - 126.7) | |
| 2007 | 103.1 | 121 (117.3 - 124.7) | -1.55 | |
| 2008 | 96 | 108 (104.5 - 111.4) | -10.74 | |
| 2009 | 98 | 105.6 (102.3 - 108.9) | -2.22 | |
| 4 years | 99 | 113.9 (112.1 - 115.6) | -0.05 | |
| Women | 2006 | 93.4 | 109.2 (105.5 - 112.8) | |
| 2007 | 95.2 | 104.9 (101.4 - 108.3) | -3.94 | |
| 2008 | 94.7 | 99.8 (96.5 - 103) | -4.86 | |
| 2009 | 91.6 | 93.6 (90.5 - 96.6) | -6.21 | |
| 4 years | 93.7 | 101.3 (99.7-103) | -0.05 |
Abbreviations: APC, annual percent change which is measured based on the ASR; ASR, age standardized rate.
3.2. Models
Over time, the trend of cancer rate decreased nonlinearly (in a quadratic form) and the model with variable year with power 2 had less AIC than other transformations of year variable; hence, we entered an ordinal variable containing power 2 of year in all models.
3.3. Regions with Different Socioeconomic Status
After grouping the districts in 4 socioeconomical different regions, region 4 in the northern area of municipality with higher socioeconomic position showed 1.31 higher incidence rate of cancer in comparison with region 1 in southern area of Tehran with lower socioeconomic status among both sexes (Table 2). We also checked the interaction of calendar year of investigation and region. The results showed a partially significant interaction with higher decrease in annual trend of cancer rate in socially more deprived regions.
| Total Cancer | Men | Women | Both Sexes | ||||
|---|---|---|---|---|---|---|---|
| IRR (CI95) | P Value | IRR (CI95) | P Value | IRR | P Value | ||
| Year 2 | 0.99 (0.98 - 0.99) | < 0.001 | 0.99 (0.98 - 0.99) | < 0.001 | 0.99 (0.98 - 0.99) | < 0.001 | |
| Regions | 1.1 (1.07 - 1.13) | < 0.001 | 1.04 (1 - 1.08) | 0.05 | 1.07 (1.05 - 1.09) | < 0.001 | |
| Age group | 10 - 14 | 0.19 (0.12 - 0.3) | < 0.001 | 0.1 (0.07 - 0.13) | < 0.001 | 0.06 (0.05 - 0.08) | < 0.001 |
| 15 - 19 | 0.27 (0.19 - 0.39) | 0.03 | 0.16 (0.13 - 0.2) | 0.12 | 0.11 (0.09 - 0.12) | < 0.001 | |
| 20 - 24 | 0.31 (0.24 - 0.39) | < 0.001 | 0.13 (0.11 - 0.16) | < 0.001 | 0.14 (0.12 - 0.16) | < 0.001 | |
| 25 - 29 | 0.4 (0.34 - 0.47) | < 0.001 | 0.17 (0.15 - 0.19) | < 0.001 | 0.24 (0.22 - 0.27) | < 0.001 | |
| 30 - 34 | 0.49 (0.42- 0.57) | < 0.001 | 0.29 (0.26 - 0.33) | 0.03 | 0.38 (0.34 - 0.42) | < 0.001 | |
| 35 - 39 | 0.69 (0.6 - 0.79) | 0.19 | 0.54 (0.48 - 0.6) | 0.47 | 0.62 (0.56 - 0.68) | < 0.001 | |
| 40 - 44 | 1 | - | 1 | - | 1 | - | |
| 45 - 49 | 1.66 (1.47 - 1.88) | 0.26 | 1.73 (1.57 - 1.9) | 0.15 | 1.55 (1.42 - 1.68) | < 0.001 | |
| 50 - 54 | 3.26 (2.82 - 3.76) | 0.44 | 2.78 (2.46 -3.14) | 0.67 | 2.33 (2.15 - 2.53) | < 0.001 | |
| 55 - 59 | 5.61 (4.65 -6.77) | < 0.001 | 4.15 (3.56 - 4.84) | < 0.001 | 3.31 (3.05 - 3.6) | < 0.001 | |
| 60 - 64 | 9.7 (7.59 - 12.41) | 0.39 | 5.85 (4.9 - 6.99) | 0.67 | 4.85 (4.45 - 5.28) | < 0.001 | |
| 65 ≤ | 19.01 (13.79 - 26.2) | 0.77 | 8.52 (6.88 - 10.55) | 0.77 | 8.54 (7.77 - 9.38) | < 0.001 | |
| Literacya | 1.06 (1 - 1.11) | 0.04 | 1.13 (1.09 - 1.18) | < 0.001 | 1.22 (1.18 - 1.25) | < 0.001 | |
| Employmenta | 1.09 (0.95 - 1.25) | 0.23 | 2.08 (1.68 - 2.57) | < 0.001 | 0.81 (0.79 - 0.83) | < 0.001 | |
aThe effect is estimated for one standard deviation change in the proportion
We also estimated the effect of living in each district on cancer mortality rate (The detailed results and tables are presented in the appendix.). District 16 with the least incidence in south center of Tehran (tables in the supplementary file) and age group 40, which is the changing point, were considered baseline, and incidence rate ratios were reported compared to theses base lines.
The total number of observation was 30,827, but the living location of 2,441 persons was missing. The results of model 0, which was fitted on 30,827 records, are presented in Table 3.
| Total Cancer | Men | Women | |||
|---|---|---|---|---|---|
| Incidence Rate Ratio (CI 95%) | P Value | Incidence Rate Ratio (CI 95%) | P Value | ||
| Year 2a | 0.99 (0.99 - 0.99) | < 0.001 | 0.99 (0.99 - 0.99) | < 0.001 | |
| Age group | 0 | 0.27 (0.22 - 0.32) | < 0.001 | 0.12 (0.1 - 0.14) | < 0.001 |
| 5 | 0.1 (0.07 - 0.13) | < 0.001 | 0.05 (0.03 - 0.06) | < 0.001 | |
| 10 | 0.15 (0.12 - 0.19) | 0.03 | 0.06 (0.05 - 0.08) | 0.12 | |
| 15 | 0.22 (0.18 - 0.26) | < 0.001 | 0.11 (0.09 - 0.13) | < 0.001 | |
| 20 | 0.28 (0.24 - 0.33) | < 0.001 | 0.12 (0.1 - 0.13) | < 0.001 | |
| 25 | 0.37 (0.33 - 0.43) | < 0.001 | 0.19 (0.17 - 0.21) | 0.03 | |
| 30 | 0.48 (0.42 - 0.55) | 0.19 | 0.33 (0.3 - 0.37) | 0.47 | |
| 35 | 0.68 (0.6 - 0.76) | 0.26 | 0.59 (0.54 - 0.64) | 0.15 | |
| 40 | 1 | - | 1 | - | |
| 45 | 1.66 (1.49 - 1.85) | 0.44 | 1.55 (1.43 - 1.66) | 0.67 | |
| 50 | 3.12 (2.83 - 3.44) | < 0.001 | 2.09 (1.94 - 2.25) | < 0.001 | |
| 55 | 5.17 (4.71 - 5.69) | 0.39 | 2.69 (2.5 - 2.89) | 0.67 | |
| 60 | 8.42 (7.68 - 9.24) | 0.77 | 3.37 (3.13 - 3.63) | 0.77 | |
| 65 | 14.64 (13.45 - 15.92) | < 0.001 | 3.94 (3.7 - 4.2) | < 0.001 | |
aYear power 2 (1 power 2 for 2006 and 4 power 2 for 2009).
In model 1 that district was entered (appendix 1, 2 in the supplementary file), the incidence rate (IR) varied according to the districts. The lowest rate ratio was attributed to district 17 and 18 located in the south of Tehran. The highest IRR belonged to district 6 for both sexes. The IRR in this district was 72% and 90% higher compared to district 16 in men and women respectively, but after adjustment for literacy and employment, the rate decreased to 59% and 37%. It seems that literacy and employment rate are strong confounders in this model with more impact in women in comparison to men.
Northern districts of Tehran (district number 1 to 7) and 2 other ones, district 11 in center and district 20 in south of Tehran, had higher incidence compared to district 16. Other districts, which were located mostly in south of Tehran had lower incidence in both sexes.
The IRR significantly increased with increasing age groups. This pattern was almost similar among both sexes, but slope of changes was sharper in men compared to women. According to the model 3, literacy proportion was positively associated with cancer incidence. The incidence rate ratio for each standard deviation increase in the literacy proportion was 5% and 9% for men and women, respectively. Employment rate did not impact on the risk of cancer among men on the contrary; among women, each 1 unit increase in standard deviation of employment proportion was significantly associated with 1.6 times the risk of cancer (appendix 1, 2 in the supplementary file).
4. Discussion
The findings of the present study showed that total cancer incidence was 114 and 101 per 100 000 men and women, respectively in Tehran during 2006 to 2009. Both APC based on ASR and regression model showed a decreasing trend. In this study, both sexes showed decreasing trend, but incidence trends in some cities have increased significantly (26-28); in European countries, incidence trends, in males, are decreasing, while for females, they go on to ascent (29-31).
According to the global pattern of cancer incidence and trend study, from 1998 to 2002, the rate of cancers was decreasing in many western countries, but this rate in less developed and Eastern European countries was increasing because of changes in lifestyle pattern (32).
According to the annual report of Tehran pathologic based cancer registry during 1998 to 2001, which revealed information on cancer incidence for Tehran plus Eslamshahr and Shemiranat (with population around 5% of Tehran), the ASR was 121 and 106 for men and women, respectively. The ASR rates in the current study from 2006 t0 2009 were 114 and 101 for men and women, respectively. Although in the current study, Eslamshar and shemiranat cities are not included but even after adjusting the rate according to the population of this city, comparing the results shows a decrease in the male total cancer rate in Tehran (33).
Join point regression analysis of data of New York state cancer registry, which reported all invasive cancers, showed an increasing trend of cancer incidence from 1976 to 1992 and decreasing trend from 1992 to 2009 in men. This study also exhibited an increasing trend of invasive cancers from 1976 to 1998 and a decreasing trend from 1998 to 2009 among women. AAPC in this study was reported -0.1 in both sexes from 2000 to 2009 (32-34).
These changes in cancer incidence were also reported in other studies. Surveillance data for cancer prevention and control in the United States from 1975 to 2000, which reported overall cancer incidence rates depicted an increasing trend from the mid-1970s through 1992 and decreasing trend from 1992 through 1995, which became stable afterwards until 2000 (2).
Overall cancer incidence trend may cover important trend changes in different cancers. For example, in the United Kingdom from 1993 to 2003, the rate of prostate, oral, melanoma increased, but the rate of male lung cancer, stomach, and bladder, and cervix cancers decreased. In this period, colorectal and female lung cancer rate were relatively stable (3). The analysis of cancer trend based on population-based cancer registries in 4 selected prefectures in Japan between 1985 and 2007 reflected that overall cancer incidence increased with an APC of 0.7%. The incidence rate of prostate cancer increased until 2000 with an APC of 5.1%, obviously rise from 2000 to 2003 with an APC of 29.7%, and became stable, thereafter. Liver, colorectal, lung (males), and female breast cancer showed consecutively a pattern of upward trend, while Stomach cancer showed downward trend from 1985 with APCs of -1.7% and -2.5% for males and females, respectively (35).
In line with New York and United States studies, the present study showed a decreasing trend of total cancer incidence from 2006 to 2009 in Iran, but this trend is trivial. Incidence rate also differed among districts. The northern and central districts had higher incidence than southern districts. The rate of total cancers across district 6 (in the northern area of Tehran) was 60% and 37% higher than district 16 (which was located in south of Tehran) between men and women, respectively. Generally, districts located in the region 4 in the northern area of municipality with higher socioeconomic position showed higher incidence rate of cancer in comparison with districts, which were located in the region 1 in southern area of Tehran with lower socioeconomic status. The results also demonstrated higher decrease in annual trend of cancer rate in socially more deprived regions. A number of probable factors contribute to these geographical differences such as uneven distribution of risk factors, availability of medical services, socioeconomic status differences, and healthy lifestyle changes (36, 37).
In economically developed countries, 78% of cancer incident cases occur at age 55 and older compared to 58% in developing countries; in our study, 51% of cancers occurred at age 55 and older. The difference is largely due to variations in age structure of the populations (38).
Incidence rate ratio comparing cancer incidence of different age groups to the baseline age group of 40 to 45 years exhibited a sharp rise in men after the age of 65. This is probably due to the increasing rate of prostate cancer in this age group. Such a rise was not observed in elderly women because of steady distribution of breast cancer (8, 39).
In our study, employment and literacy rate had a positive impact on the risk of cancer among women. In a study of cancer incidence among residents in Turin during 1985 to 1999, lower education level and low occupation class were associated with the higher risk of overall cancer among men. On the contrary, among women, higher education level was associated with higher risk (RII = 0.78, 95% CI: 0.72 - 0.85). In this study, occupation was not significantly associated with the risk of cancer (40). Some studies also support the association of different socioeconomic factors with the rate of different cancers or their risk factors (41). For instance, a study across Iran’s provinces from 2003 to 2009 investigated social disparities in breast cancer (BC) and ovarian cancer (OC) incidence rates. In this study, the incidences of BC and OC were higher across the provinces with higher social rank based on human development index (10, 12, 42, 43). Based on European incidence rates by deprivation quintile england from 2006 to 2010, all cancers combined ASR excluding non-melanoma skin cancer were higher in the most deprived than the least deprived groups and this is similar for both sexes. Few cancers have more cases in less deprived, for example, female breast and prostate cancer (39). This association might be partly explained by higher prevalence of risk factors in this group (9, 44) or more surveillance among women with higher social rank (45, 46). These results indicate the effects of underlying some risk and protective factors on different cancer incidence trend and necessity of analysis for different cancer types separately.
5. Conclusions
The results showed downward and nonlinear decreasing trend during 4 years, especially in regions with lower socioeconomic status. Incidence rate also differed between districts so that northern region had higher incidence than southern region. Spatio-temporal Analysis of theses cancer rates with adjustment for more district and regional socioeconomic characteristics may reveal this disparities in rate of cancer in different districts across time. It should also be mentioned that all associations in this study are estimated according to group data and individual inference of these associations should be done precautiously to avoid ecological fallacy.