1. Introduction
EGFR (Epidermal growth factor receptor), also known as HER-1 or c-erbB-1, is a 170-kDa glycoprotein that consists of 1186-residue protein (1). It is the first member of the ErbB family which also consists of HER2 (c-erbB-2), HER3 (c-erbB-3) and HER4 (c-erbB-4) (2). EGFR is inserted into the cell membrane and consists of an extracellular N-terminaldomain which is ligand-binding region, a hydrophobic transmembrane region and also an intracellular domain which has tyrosine kinase activity (1, 3).
EGFR activation modulates numerous biological processes including gene expression, proliferation of cell, angiogenesis, and inhibition of apoptosis leading to development of malignancy, invasion and metastasis. EGFR activation also causes invasion, adhesion and metastasis of tumor cells (4). EGFR overexpression plays an important role in aggressiveness of some cancer types such as head and neck cancer (5), renal cancer (6), non-small cell lung cancer (7), ovarian cancer (8) and colon cancer (9). Increased EGFR expression in these tumors have been contributed to less survival, resistance to hormone therapy, chemotherapy and also radiation (10). Several selective agents, targeted against EGFR were developed and clinically approved by food and drug administration (FDA). These agents consist two major groups; the first group is small molecule tyrosine kinase inhibitors which act against intracellular portion of the receptor and inhibit its tyrosine kinase activity. Various EGFR tyrosine kinase inhibitors have been produced with their newer generations having more specificity for receptors, therefore, resulting in a greater antitumor property (11).
Gefitinib is an oral inhibitor of EGFR tyrosine kinase activity which has shown good antitumor activity in treatment of non-small-cell lung cancer (12) and also head and neck squamous cell carcinoma (13). Erlotinib is another tyrosine kinase inhibitor which has shown antitumor activity in head and neck tumor cell lines, metastatic tumor mass in lung of a patient with primary renal cell carcinoma and is used as a first line treatment of metastatic non-small cell lung cancer with certain EGFR expression (14).
The second group of selective agents consist of monoclonal antibodies that act directly against extracellular domain of the receptor and block its activation by ligands (15). Cetuximab is a recombinant monoclonal antibody which is used for treatment of metastatic colon cancer (16). Panitumumab, a monoclonal antibody which acts against extracellular domain of the EGFR, is applied in metastatic colorectal cancer, head and neck and lung cancer patients who had failed to improve with routine chemotherapy agents (17). Since these drugs are covering limited ranges of solid tumors and considering the difficulties in production and using monoclonal antibodies (18), the search for new therapeutic agents is still ongoing.
Being the smallest functional part of immunoglobulin, Fv fragment can have some advantages over murine antibodies; better and more efficient penetration to target tissues (19), higher specificity and affinity for antigens (20-22), easier and more efficient ways of production, more rapid clearance from circulation due to its small size and also being less immunogenic due to production of antibodies from human genome libraries (18, 23).
In this study, phage antibody and panning process were used to select scFvs against an immunodominant epitope of EGFR. The selected antibodies were evaluated in ELISA.
2. Objectives
The aim of this study was to produce specific human single-chain antibodies against EGFR and evaluate of its specificity against the epitope in order to offer a new and efficient way in the treatment of EGFR expressing tumor tissues.
3. Methods
3.1. Phage Rescue
As described previously, a phage antibody display library of scFv was produced (24, 25). Escherichia coli bacteria containing phagemid were cultured on 2TYG agar/ampicillin (tryptone, yeast extract, glucose, agar and ampicillin) (Merck, Germany) plates overnight at 30°C. The bacteria were scraped and incubated in 2TYG broth at 37°C for 1 hour. Helper phage (M13KO7) was added and incubated at 37°C for 30 minutes. The culture was shaken for 30 minutes and centrifuged at 3500 rpm for 20 minutes. The bacterial pellet was transferred to 2TY broth containing ampicillin (100 μg/mL) and kanamycin (50 μL/mL) and incubated with shaking at 30°C overnight. The culture was centrifuged at 5500 rpm and the supernatant was passed through 0.2 μm filters and stored at 4°C.
3.2. Panning
Peptide (LYNPTTYQMD) at a concentration of 10 μg/mL was coated on polystyrene immuno-tubes (Nunc, Denmark) at 4°C overnight. The tubes were washed four times with phosphate buffered saline (PBS), and blocking solution (2% skimmed milk) was added and incubated at 37°C for two hours. The tubes were washed four times with PBS/ Tween and four times with PBS. The diluted phage supernatant in the blocking solution (1/1) was added to the tubes and incubated at room temperature for one hour with occasional inversions. The tubes were washed and logarithmic phase E. coli was added, incubated at 37°C for one hour and centrifugation at 3500 rpm was done. The bacterial pellet was re-suspended in 2TY broth (tryptone, yeast extract) (Merck, Germany), plated on 2TY agarose/ ampicillin plate and incubated at 30°C, overnight. Four rounds of panning were performed to select specific scFv antibodies against the epitope.
3.3. DNA Fingerprinting of the Selected Clones
The inserts of selected clones were amplified by PCR (94°C for one minute, 55°C for one minute and 72°C for two minutes, performed in 30 cycles). To demonstrate the fingerprinting patterns, 17 μL of the PCR product was mixed with 1 μL restriction enzyme (Mva-I) (Roche Applied Science, Germany) and 2 μL of restriction enzyme buffer. The mixtures were placed in a dry block heater at 37°C for two hours and ran on a 2% agarose gel.
3.4. Phage ELISA
The peptide (100 μg/mL) as an epitope was coated in 96 wells of a polystyrene plate and incubated at 4°C overnight. After washing with PBS, blocking solution (2% skimmed milk) was added to each well and incubated at 37°C for two hours. The wells were washed with PBS/Tween and PBS. Phage rescue supernatant containing the appropriate scFv, diluted with blocking solution (1:1), was added to each well and incubated at room temperature for two hours. Wells were washed three times with PBS/Tween, then three times with PBS to remove the non-bound phages. Anti-Fd bacteriophage (1:100) (Sigma, Germany) was added to each well and incubated at room temperature for 1.5 hours. HRP-conjugated anti-rabbit antibody (1:1000) (Sigma, Germany) was added to each well and incubated at room temperature for one hour. The wells were washed and Azino-bis-3-ethylbenzothiazoline-6-sulfonic (ABTS) acid (Sigma-Aldrich, Germany) solution (10 mg ABTS, 20 mL citrate buffer PH4 and 6 mL H2O2) was added to each well. The absorbances were read at 405 nm after 30 minutes using an ELISA reader. The wells without peptide, with unrelated peptide (prostate stem cell antigen peptide), unrelated scFv (scFv to HER2), and with M13KO7 were also considered as negative controls.
4. Results
4.1. PCR and DNA Fingerprinting
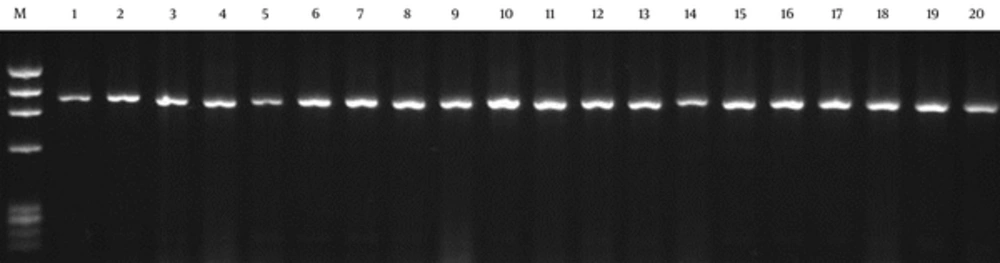
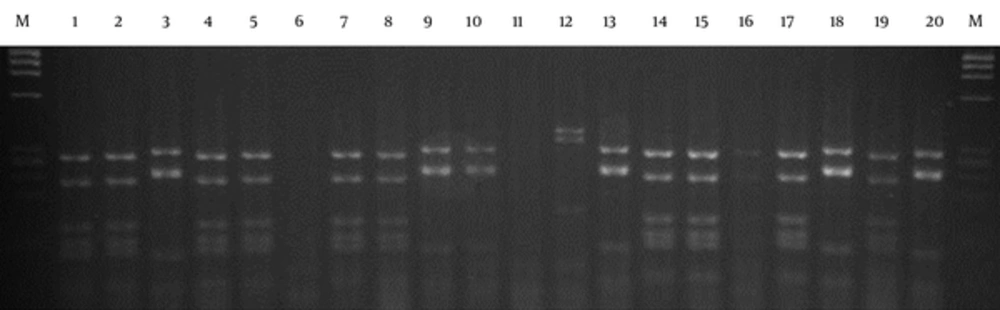
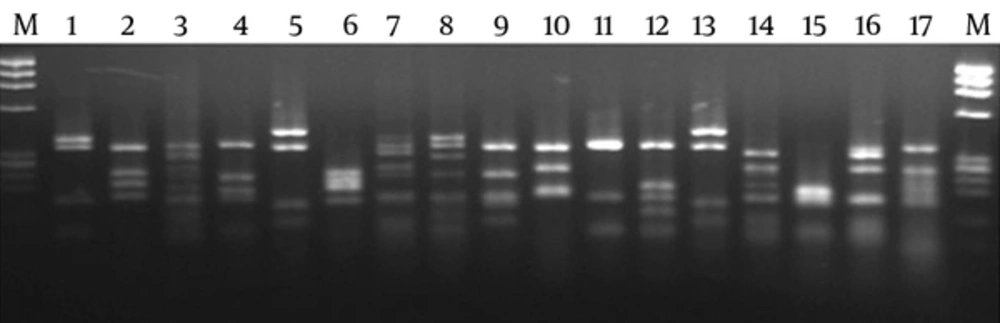
Figures 1 and 2 show the PCR and DNA fingerprinting of 20 clones after the panning process, respectively. The 950 bp band (VH-linker-VL) was obtained for all the clones (Figure 1). Two dominant fingerprinting patterns were obtained. Pattern 1, scFv1, (lanes 1, 2, 4, 5, 7, 8, 14, 15, 16, 17 and 19) with frequency of 55% and pattern 2, scFv2, (lanes 3, 9, 10, 13, 18 and 20) with frequency of 30% were chosen. One clone from each pattern was selected for further evaluation (Figure 2). The fingerprinting of 20 clones from the library is demonstrated in Figure 3. The patterns show the diversity and heterogeneity of the library before panning.
4.2. Phage Enzyme Linked Immunosorbent Assay
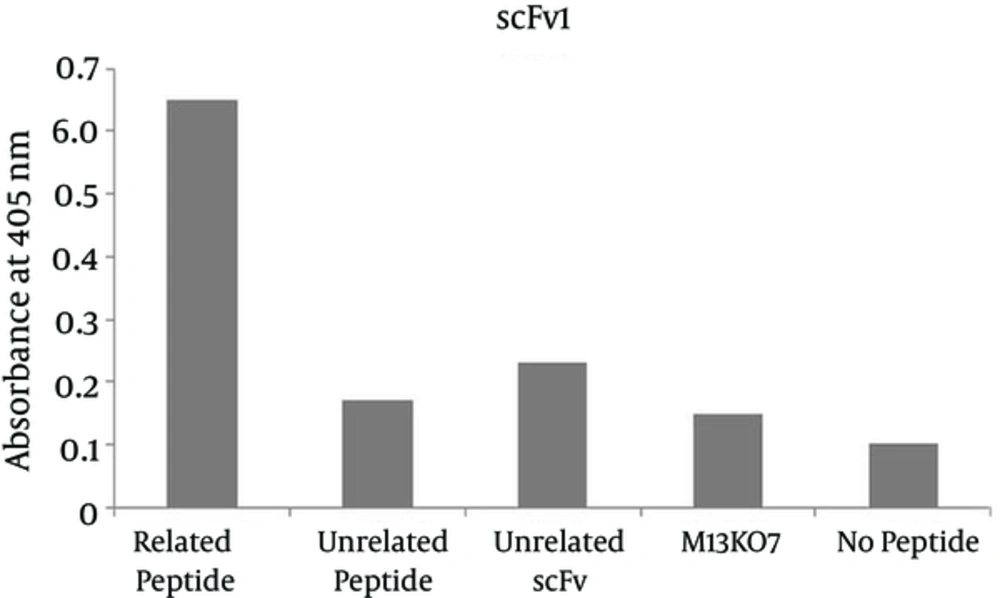
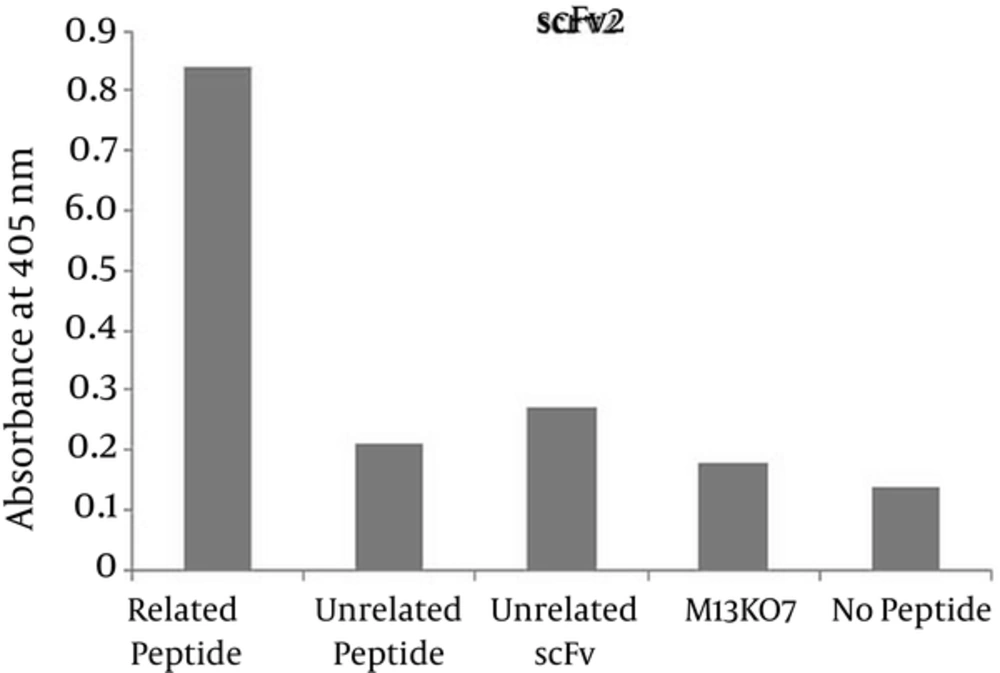
Phage ELISA was used to demonstrate the specific binding of the selected scFv antibodies to the corresponding peptide. The absorbance of wells coated with the corresponding peptide for the two selected scFvs were significantly higher than that of the wells containing no peptide, measured at 0.65 and 0.84 for reaction of scFv1 and scFv2 with the corresponding epitope versus 0.10 and 0.14 for no peptide wells respectively. The ODs at 405 nm are shown in Figures 4 and 5.
5. Discussion
Lots of effective treatments such as chemotherapy, radiotherapy, surgery and immunotherapy were developed for treating malignancies. Although these treatments are successful, they lack selectivity in targeting malignant tissues which causes them to exert their effect on other tissues apart from tumoral tissues and cause disadvantageous side effects such as trauma, immunosuppression, cosmetic damage and systemic toxicity. New era in treatment of cancer was established by the name of “targeted therapy” in which the molecular pathways of tumoral cell proliferation and propagation are manipulated by attachment of highly specific antibodies to selected antigens. Multiple monoclonal antibodies have been made to target EGFR. Cetuximab (Erbitux) is a chimeric antibody which functions against extracellular portion of EGFR receptor and results in inhibition of tumor growth and also reduction of its mass. This drug has some important adverse effects such as Infusion reactions, acneiform eruptions and nail disorders (26). Panitumumab (Vectibix), another monoclonal antibody specific against EGFR, is a fully humanized IgG2 monoclonal antibody which also acts against the extracellular part of EGFR and inhibits receptor’s tyrosine phosphorylation, cell proliferation, and angiogenesis of EGFR-expressing cells. Panitumumab has higher affinity to EGFR than its ligands such as EGF and TGF-α (27). Since monoclonal antibodies have several disadvantages such as being expensive, consuming lots of time for production, not making the highest affinity against the target, having less penetration due to big size, and having non-human parts, their function have been limited in practice. Although some humanized monoclonal antibodies have been produced, still HAMA response (human anti-mouse antibody response) occurs which is due to CDR parts of antibody which cannot be changed.
Single chain antibodies have several desirable advantages over monoclonal antibodies such as better penetration due to their smaller size, human origin, being easily made, and high affinity and specificity which made them attractive alternatives to monoclonal antibodies in cancer immunotherapy (18, 28-30).
Although production of single chain antibody is a new way in treatment of diseases, there are several scFvs in clinical trials. ESBA-105 is an anti-TNFα scFv which has passed phase 2 clinical trial successfully for treatment of ophthalmic diseases (31). Efungumab (Mycograb) is another scFv in phase 2 clinical trial which can help treatment of invasive candidiasis (32). ScFv (FRP5)-ETA is a toxin-bound scFv with binding specificity for ErbB2 (HER2) which has passed phase 1 clinical trial in the treatment of advanced solid malignomas (33). There are several scFvs which were tested in vitro and showed encouraging results. Nejatollahi et al. showed that the mixture of the three anti-HER2 scFvs inhibits proliferation of breast cancer cells and downregulates HER2 gene and protein expression (20).
In this study we selected two specific single-chain antibodies against an immunodominant portion of EGFR antigen, amino acids 245 - 254 of EGFR molecule (LYNPTTYQMD). The ligand-binding region of EGFR molecule consists of four domains that are called L1, CR1, L2, and CR2 domains which are also referred to as domainsI-IV (34). The immunodominant epitope applied in this study is a part of domain II of the EGFR molecule and plays an important role in dimerization and subsequent phosphorylation, signaling, cell proliferation and differentiation. Polyclonal rabbit anti serum was generated against polypeptide LYNPTTYQMD. In order to produce anti-EGFR monoclonal antibody, this peptide was injected to rabbit and the resulting antiserum was collected. The purified antibody was used for treating human mammary epithelial cells. The study showed that High doses of antibody, normalizes the tyrosine phosphorylation; Therefore, antibodies binding to LYNPTTYQMD can decrease the tyrosine phosphorylation of EGFR (35).
EGFR is activated by several ligands including epidermal growth factor (EGF), transforming growth factor, heparin-binding EGF and betacellulin (36). The most important ligands are EGF and transforming growth factor. Binding of ligands to the receptor causes homo or heterodimerization and then internalization of the dimerized receptors to the cell. After all these events, autophosphorylation of intracytoplasmic domain of EGFR happens, which causes stimulation of intracellular signal transduction cascade (37). Antibodies against EGFR can inhibit the process which leads to decrease of cancer growth.
The results of panning process demonstrated two specific antibodies, scFv1 and scFv2 with frequencies of 55% and 30% respectively. A number of studies have shown the selection of specific scFvs against different targets by panning process. In 2013 two antibodies against two immunodominant epitopes of Prostate stem cell antigen (PSCA) were selected by Nejatollahi et al. using panning process and their reactivity were determined by phage ELISA (38). Younesi et al. isolated scFvs against two specific epitopes of IL-25R by performing panning process on a phage library and assessed their specificity by phage ELISA (39). Luo et al. produced scFvs against Prx I, an anti-apoptosis protein for tumor cell proliferation and survival which is overexpressed in lung adenocarcinoma tumor cells, using the panning process and screened performance of clones for binding to Prx I by phage ELISA and (40). The panning results were also confirmed by ELISA in our study. In the ELISA results, it is shown that the two selected significantly higher than the negative control, no peptide well. Moreover the antibody controls, unrelated scFv and M13KO7 showed no reactivity with the epitope. The unrelated scFv (scFv to HER2) didn’t react with the EGFR peptide. The absorbances of wells coated with peptide of interest for the selected scFv1 and scFv2 were 6 and 6.5 folds higher than that of the wells containing no peptide respectively. The specificity of the selected scFvs to the corresponding peptides has been shown in various phage-ELISA assessments. Thathaisong et al. (41) showed that the optical density (OD) of specific scFvs against influenza-A virus H5N1 subtype at 405 nm was two folds higher than the negative controls in a positive phage ELISA. No reactivity of the selected scFvs with the unrelated peptide was obtained which represents the specific reaction of the selected scFvs with the corresponding peptide.
EGFR is expressed on many cancerous cells and is a potential marker for immunotherapy (42, 43). The specific anti-EGFR scFvs selected in this study offer new agents with high affinity for targeted therapy. These antibodies not only don't produce human anti-mouse antibody (HAMA) response but also can be manipulated and used as a cargo for delivery of drugs, radioisotopes, toxins, and enzymes. Furthermore, they could also could interfere with cellular processes by binding to extracellular portion of EGFR and exert anti-tumor activity against head and neck, renal, non-small cell lung, and colon cancers. In order to develop these functions, further investigations are needed to evaluate the effects of these antibodies in vitro and in vivo.