1. Background
The global burden of colorectal cancer (CRC) is expected to increase by 60%, to more than 2.2 million new cases, and 1.1 million deaths by 2030 (1). CRC is the most common cancer among gastrointestinal malignancies and the fourth leading cancer-related death in the world (2). The gastrointestinal cancers are the most frequent cancer among Iranian males and the second among females (3). The majority of the GI cancers occur in the stomach, the ninth colon and rectum (4). Although colorectal cancer is a public health burden in most industrialized countries (1), its burden is increasing in the Iranian population (5) and according to an Iranian study, there is a younger age distribution for CRC compared to Western reports (6).
During the past decades, survival of colorectal cancer patients has improved worldwide, but the clinicians who encounter patients with CRC need to know the expected prognosis regarding patients’ survival to plan appropriate therapies (7). Survival analysis is the analysis of data measured from a specific time of origin until an event of interest or a specified endpoint (8). According to the general model of survival, every patient provides two pieces of information: follow-up time and status (dead or alive). There are situations in which a studied person can experience one of several different types of events. On the other hand, the patient may die due to causes unrelated to the main disease. Such events are termed competing risk events (9).
Researchers in medical sciences often tend to prefer semi parametric over parametric models because they require less assumptions (10). When competing events are present, Cox regression and Kaplan-Meier method are invalid because a subject who has failed in other competing risks is treated as a censored subject. In the process of censoring, the main event still happens at a later time but maybe could not be observed at the time of happening. Although Cox regression modeling and Kaplan-Meier method are popular techniques for survival analysis, ignoring competing risks causes bias in the model results. The susceptibility of such analyses to biased estimates when competing events are present may be less known (11). In 2016, van Walraven and McAlister examined 100 studies with Kaplan-Meier estimates that were recently published in high-impact medical journals and found that forty-six studies (46%) were susceptible to overestimated risks (12). So alternative methods specifically designed for analyzing competing risks data that consider competing events (such as parametric models) should then be applied (13). When these parametric models provide a good fit to data, they tend to give more precise estimates of the quantities of interest because these estimates are based on fewer parameters (14).
In this study, we focus on the survival analysis by Generalized Weibull model for competing risks data of colorectal cancer. Generalized Weibull distribution is a distribution with an extra parameter (compared to classic Weibull), which could be more flexible for analyzing the competing risk model due to its ability to cover different types of hazard functions (15).
The aim of this study was to assess the association between survival of patients with colorectal cancer and prognostic factors in a competing risk parametric model using generalized Weibull distribution.
2. Methods
Data were provided from Taleghani hospital, Tehran, Iran in a retrospective cohort study. A total of 1462 patients with colorectal cancer who registered in cancer registry center of research institute of gastroenterology and liver disease were referred to Taleghani hospital between January, 2004 and January, 2014. The patients were followed up, until April 2015, and their survival status was identified. Of them, 402 patients were omitted due to incomplete information of non-specific survival time. This study was approved by the ethics committee at Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences.
Death and the causes of death were confirmed via telephone contact to patients’ families, which is an official plan of cancer department in research institute of gastroenterology and liver disease, and each year one or two times the last situation of all registered patients is followed through telephone contact. In some cases with skeptical information, the telephone contact is repeated to assure the accuracy of information. Mortality due to CRC is considered as the main event in survival model and other causes of death are considered as the competing risks. All patient deaths during this period were considered as a consequence of the colorectal cancer. The demographic and clinical features which were extracted from hospital documents included age at diagnosis, sex, family history of CRC, body mass index (BMI), tumor size and tumor site. BMI values (in kg/m2) were grouped into the four world health organization categories (16): underweight (BMI < 18.5), normal (18.5 - 24.9), overweight (25.0 - 29.9), and obese (≥ 30.0). Because the survival curves were matched for patients who were with a BMI less than 18.5 and people with a BMI between 18.5 and 24.9; therefore, we have integrated the two categories.
Prognostic variables were entered in a parametric model called generalized Weibull distribution (15), in order to analyze the survival and its prognosis, in the presence of competing risk. Also Weibull model and Fine and Gray model (as proportional hazards semi parametric model for competing risks) were employed to analyze the data. Besides, Cox regression analysis was done without considering the competing risks. The program of Generalized Weibull model in the presence of competing-risks has been written in the R software (version 3.0.3.) using package foreign and function optim. STATA software (version11) was used for Cox and Gray models. P value less than 0.05 were considered significant.
3. Results
Overall, 1060 CRC patients were included in the analysis. 615 patients (58%) were men and the mean ± SD of age at diagnosis was 53.67 ± 0.46 years (range: 12 - 97 years). The survival time from diagnosis to the events (death from CRC or other risk factors) or until the end of the study for patients who were still alive (as the right censored) was considered as month and the mean ± SD of survival time was calculated 56.96 ± 1.46 with median = 45.5 months (range; 1 - 356 months).
In recent follow ups, it is found that 380 patients (35.5%) died from CRC and 49 patients (4.6%) died from other causes of death, such as myocardial infarction, stomach cancer, liver cancer etc. The median of survival for patient who died from CRC was 33 ± 1.83 months (95% CI; 29.5 - 36.5). The mean ± SD of BMI was 24.5 ± 0.13. The clinical and demographic variables are in Table 1, indicating that 51.6% of tumor sites were in rectum, 82.8% of tumor size was more than 1 cm, and 56.8% of patients reported family history of CRC in their relatives (Table 1).
| Variables | Number (%) | Death Due to CRC | Death Due to Other Risks | Mean of Survival Time (SD) |
|---|---|---|---|---|
| Tumor Site | ||||
| Colon | 513 (48.4) | 194 (51.1) | 23 (46.9) | 60.5 (2.8) |
| Rectum | 547 (51.6) | 186 (48.9) | 26 (53.1) | 53.5 (1.84) |
| Sex | ||||
| Female | 445 (42) | 144 (37.9) | 18 (36.7) | 58.8 (2.19) |
| Male | 615 (58) | 236 (62.1) | 31 (63.3) | 55.6 (1.95) |
| Tumor Size, cm | ||||
| < 1 | 182 (17.2) | 64 (16.8) | 2 (4.1) | 69.19 (4.15) |
| > 1 | 878 (82.8) | 316 (83.2) | 47 (95.9) | 54.4 (1.52) |
| Family History | ||||
| Yes | 458 (43.2) | 162 (42.6) | 15 (30.6) | 59.1 (2.31) |
| No | 602 (56.8) | 218 (57.4) | 34 (69.4) | 55.3 (1.87) |
| BMI | ||||
| < 24.9 | 590 (55.6) | 245 (23.2) | 283 (6.1) | 115.58 (10.93) |
| 25 - 29.9 | 362 (34.2) | 104 (9.8) | 19 (1.8) | 201.08 (10.49) |
| > 30 | 108 (10.2) | 31 (2.9) | 1 (0.1) | 240.36 (19.60) |
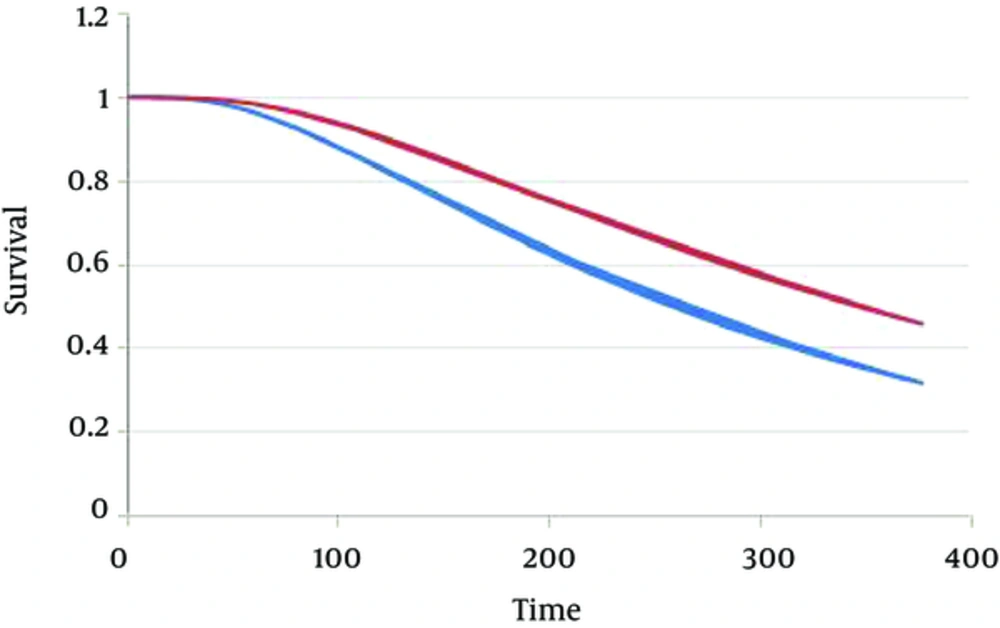
Results of survival analysis in Generalized Weibull model and Weibull model, with and without considering competing risks are presented in Tables 2 and 3. According to the competing risk model, age at diagnosis and BMI were the only prognosis of CRC survival in patients under study. According to the results of generalized Weibull model, it was found that people with a BMI between 25 and 29.9, and People with a BMI higher than 30 were less susceptible to death from colorectal cancer than people with a BMI less than 25 (Tables 2 and 3). Figure 1 presents the survival plot of CRC patients according to Generalized Weibull competing risk model, which considered cut off 53 years old as cut off for patients (adjusted for BMI), indicating lower survival for patients who were above 53 (Graph 1). Other factors such as tumor site, tumor size, sex and family history of CRC had no significant association to survival time of patients with CRC, not only in Weibull models, but also in both Cox proportional model (Table 4) and Fine-Gary model (Table 4). Age at diagnosis and BMI were still significant prognosis factors according to those models too.
| Variables | Generalized Weibull Model | Weibull Model | ||||
|---|---|---|---|---|---|---|
| Coefficient (SE) | HR (95% CI) | P Value | Coefficient (SE) | HR (95% CI) | P Value | |
| Constant | -1.514 (0.021) | 0.220 (0.212, 0.227) | < 0.001* | -5.88 (0.055) | 0.002 (0.002, 0.03) | < 0.001* |
| Tumor Site | 0.60 | |||||
| Colon | -0.048 (0.031) | 0.953 (0.904, 1.004) | 0.14 | -0.042 (0.076) | 0.959 (0.825, 1.114) | |
| Rectum | - | - | - | 1 | ||
| Sex | 0.22 | |||||
| Female | -0.047 (0.033) | 0.954 (0.902, 1.008) | 0.16 | -0.019 (0.088) | 0.897 (0.754, 1.066) | |
| Male | - | - | - | - | ||
| Tumor size, cm | 0.48 | |||||
| < 1 | -0.008 (0.033) | 0.992 (0.938, 1.048) | 0.11 | -0.006 (0.133) | 0.993 (0.764, 1.291) | |
| > 1 | - | - | - | - | ||
| Family history | 0.16 | |||||
| Yes | -0.089 (0.051) | 0.915 (0.839, 0.997) | 0.10 | 0.102 (0.081) | 1.107 (0.945, 1.298) | |
| No | - | - | - | 1 | ||
| BMI | ||||||
| < 24.9 | - | - | - | 1 | ||
| 25 - 29.9 | -0.067 (0.037) | 0.935 (0.878, 0.995) | 0.04 | -0.360 (0.104) | 0.697 (0.568, 0.855) | < 0.001* |
| > 30 | -0.089 (0.066) | 0.915 (0.818, 1.022) | 0.03 | -0.642 (0.208) | 0.526 (0.350, 0.792) | < 0.001* |
| Age at diagnosis | 0.003 (0.0004) | 1.003 (1.002, 1.003) | < 0.001* | 0.018 (0.0009) | 1.019 (1.017, 1.021) | < 0.001* |
| Shape parameter (β) | 0.444 (0.005) | 1.559 (1.545, 1.572) | < 0.001* | - | - | - |
| Shape parameter (γ) | 2.127 (0.066) | 8.390 (7.504, 9.379) | < 0.001* | 0.965 (0.012) | 2.627 (2.564, 2.692) | < 0.001* |
| Variables | Generalized Weibull Model | Weibull Model | ||||
|---|---|---|---|---|---|---|
| Coefficient (SE) | HR (95% CI) | P Value | Coefficient (SE) | HR (95% CI) | P Value | |
| Constant | -2.356 (0.026) | 0.095 (0.090, 0.099) | < 0.001* | -5.752 (0.051) | 0.003 (0.003, 0.004) | < 0.001* |
| Tumor site | 0.20 | |||||
| Colon | -0.033 (0.037) | 0.968 (0.908, 1.029) | 0.20 | -0.088 (0.71) | 0.915 (0.759, 1.053) | |
| Rectum | - | - | - | 1 | ||
| Sex | < 0.001* | |||||
| Female | -0.082 (0.042) | 0.921 (0.858, 0.989) | 0.06 | -0.174 (0.083) | 0.840 (0.514, 0.989) | |
| Male | - | - | - | - | ||
| Tumor size, cm | 0.22 | |||||
| < 1 | -0.003 (0.040) | 0.997 (0.931, 1.066) | 0.47 | -0.167 (0.124) | 0.846 (0.662, 1.080) | |
| > 1 | - | - | - | - | ||
| Family history | 0.42 | |||||
| Yes | -0.118 (0.063) | 0.889 (0.798, 0.988) | 0.08 | 0.015 (0.078) | 1.015 (0.87, 1.184) | |
| No | - | - | - | 1 | ||
| BMI | ||||||
| < 24.9 | - | - | - | 1 | ||
| 25 - 29.9 | -0.167 (0.049) | 0.846 (0.778, 0.919) | 0.01 | -0.421 (0.098) | 0.656 (0.541, 0.795) | < 0.001* |
| > 30 | -0.177 (0.090) | 0.838 (0.719, 0.975) | 0.03 | -0.457 (0.179) | 0.633 (0.445, 0.900) | 0.02 |
| Age at diagnosis | 0.006 (0.0004) | 1.006 (1.005, 1.006) | < 0.001* | 0.013 (0.008) | 1.013 (1.012, 1.015) | < 0.001* |
| Shape parameter (β) | 0.519 (0.006) | 1.680 (1.663, 1.697) | < 0.001* | - | - | - |
| Shape parameter (γ) | 2.777 (0.079) | 16.071 (14.062, 18.366) | < 0.001* | 1.027 (0.011) | 2.795 (2.732, 2.859) | < 0.001* |
| Variables | Fine and Gary Model | Cox Model | ||||
|---|---|---|---|---|---|---|
| Coefficient (SE) | HR (95% CI) | P Value | Coefficient (SE) | HR (95% CI) | P Value | |
| Tumor site | 0.684 | |||||
| Colon | -0.034 (0.102) | 0.966 (-0.242 ,0.174) | 0.748 | -0.043 (0.102) | 0.957 (-0.2514, 0.166) | |
| Rectum | - | 1 | - | 1 | ||
| Sex | 0.064 | |||||
| Female | 0.194 (0.086) | .0823 (-0.406, 0.016) | 0.071 | -0.202 (0.089) | .816 (-0.415, -0.011) | |
| Male | - | 1 | - | - | ||
| Tumor Size, cm | 0.228 | |||||
| < 1 | -0.124 (0.121) | 0.883 (-0.393, -0.145) | 0.367 | -0.168 (0.117) | 0.845 (-0.4341, 1.05) | |
| > 1 | - | - | - | - | ||
| Family history | 0.886 | |||||
| Yes | 0.036 (0.110) | 1.037 (-0.172, 0.248) | 0.733 | 0.015 (0.108) | 1.015 (-0.194, 0.224) | |
| No | - | 1 | - | 1 | ||
| BMI | ||||||
| < 24.9 | - | 1 | - | 1 | ||
| 25 - 29.9 | 0.417 (0.078) | 0.658 (-0.645, -0.185) | < 0.001* | -0.410 (0.078) | 0.663 (-0.642, -0.177) | 0.001 |
| > 30 | 0.433 (0.125) | 0.641 (-0.827, -0.059) | 0.023 | -0.468 (0.120) | 0.626 (-0.846, -0.089) | 0.015 |
| Age at diagnosis | 0.010 (0.003) | 1.011 (0.003, 0.017) | 0.003 | 0.012 (0.003) | 1.012 (0.005, 0.019) | < 0.001* |
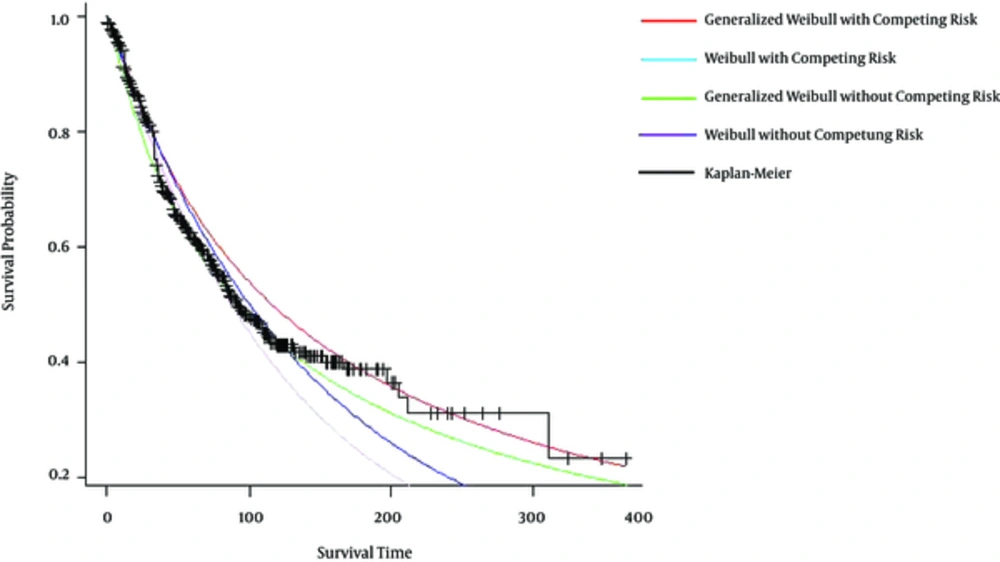
To make a comparison between two parametric models, survival probability curves based on generalized Weibull model, Weibull model with and without competing risk and Kaplan-Meier (KM) method are drawn in Figure 2. As can be seen, overall, the generalized Weibull model survival curve was closer to is Kaplan-Meier (KM) survival cure, which means generalized Weibull model has better fit compared to Weibull model distribution. Also, the 95% confidence interval for the prognostic factors based on Generalized Weibull model were shorter Compared to the confidence intervals for Cox and Gray models model (Table 4).
4. Discussion
Results from competing risk analysis with generalized Weibull model indicated that just age at diagnosis and BMI were the prognosis factors of CRC survival in patients under study. These predictors were significant in Weibull model (without considering competing risk) Cox proportional hazard regression, and Fine-Gary model too.
Age at diagnosis was a significant predictor of patients’ survival according to all models. As age increased, the rate of mortality increased. Mortality after colorectal cancer treatment may be associated with age, although evidence for this is conflicting (17, 18). This finding is in line with same study which reported age as the prognosis for CRC (19, 20) and also for both colon and rectum cancer (21). The mean age at the time of diagnosis is 53.67 years which is approximately the same as another Iranian study (22), which showed that the Iranian data still suggest a younger age distribution compared to Western reports (6, 7).
Sex was not significant according to all survival models in this study. In most countries, incidence and mortality rates are considerably higher in men than in women (23). Several studies reported superior survival in females (24, 25); while, other studies did not report any difference (26) which is similar to our results.
BMI was a prognostic factor of CRC survival in both Weibull model (with or without competing risks) and Cox and Fine-Gary model. The patients with higher BMI had better survival. A similar study suggested that underweight and obese women with colon cancer were at increased risk of death (27) and a study by Hines et al. reported higher mortality of CRC in underweight patients (28), while a recent multicenter cohort study detected little evidence for an adverse effect of excess body weight on CRC-specific survival (29).
Some studies reported better survival for colon cancer compared to rectum (30, 31). People with rectal cancer tend to be older and may have other serious health problems. In this study, tumor site and size were not significant in any models which is in contrast to the same Iranian studies (7, 32).
The last but not least potential predictor was family history of CRC. While an Iranian study showed that family history of cancer increased the risk of CRC (33), Weibull model did not detect any relation between risk of mortality and family history of CRC and this is similar to an Asian study which could not find any relation with survival of CRC and family history (34). Although in Iran there is evidence to support the screening of average risk individuals, including person with family history of colorectal cancer (35), it is still controversial and needs to more research on the Iranian population.
Although all parametric and non-parametric models in this study indicated the same significant results for age and BMI, according to survival curve, generalized Weibull model showed better fit to the data. When the parametric model has been chosen correctly, it is possible to predict the event occurrence probability in future and have a clear picture of survival time and hazard function. Also as the survival pattern follows a special parametric model, the acquired estimates are more accurate (the lower variances) than non- or semi-parametric approaches (36).
Besides, the flexibility of parametric model is beneficial for competing risk survival analysis in the case that the proportional hazards assumption is not appropriate and the shape of hazard function is not completely clear (37). The same study indicated that survival function based on parametric models, including Weibull, compared with Kaplan-Meier survival function is smooth (38, 39). This flexibility in not only for competing risk analysis, but also for classic survival analysis according to prediction error criteria (40) and based on other statistical criteria (32).
In this study, generalized Weibull was employed because of its shape parameter (as an extra parameter compared to classic Weibull) which leads to covering different types of hazard functions (15) and it is suggested to use other parametric distribution such as Log-logistic which could reflect the same flexibility as Weibull distribution to analyze the competing risk survival.
A limitation of this study is incomplete information regarding the stage of tumor. There were some data regarding the stage at diagnosis and type of treatments, but due to missing information in hospital documents, we decided to omit them from the analysis. For future studies, this information would be included in competing risk survival for better prediction.