1. Background
Gastric cancer is one of the most important causes of death from cancer in the world so much so that it was the leading cause of death from cancer in the twentieth century. Now, the incidence of gastric cancer has decreased in the western countries but it ranks second only to lung cancer. In the recent decades, the number of newly diagnosed cases of proximal gastric and esophagogastric junction (EGJ) adenocarcinomas has increased six fold and these tumors are thought to be more aggressive biologically and more complex to treat (1). Several strategies have been explained for treatment of gastric cancer in different institutes. Surgery is the standard practice in many references but lots of patients are reported unresectable before or during surgery and the local recurrence rate is high and a main problem in the operable patients (2). It seems that developments in surgical techniques have no effective role in improvement of clinical results and prognosis remains poor. Benefits of some strategies such as postoperative chemoradiotherapy (CRT) have demonstrated in decreasing local recurrence in preoperative CRT which is a new treatment method that caused promising results in treatment of proximal gastric and EGJ adenocarcinomas in several studies.
Some advantages of preoperative CRT in patients with gastric cancer include: 1) decrease in disease stage and tumor size, 2) increasing resectability rate, and 3) sterilization of the surgical field and decreasing viable tolerance and effectiveness of preoperative CRT versus postoperative CRT due to better tumor blood supply, oxygenation and smaller radiation therapy (RT) field size in preoperative setting (3). In this clinical trial, we tried to evaluate resectability and pathologic response rates of the locally advanced proximal gastric and EGJ adenocarcinomas to preoperative or neoadjuvant concurrent CRT and evaluation of toxicity profile.
2. Methods
2.1. Patient Selection
This study was approved by the ethics committee at faculty of medicine, Mashhad University of Medical Sciences. All patients with locally advanced proximal gastric or EGJ adenocarcinoma who signed the informed consent form were enrolled to the study. Definition of locally advanced tumor was according to the 7th edition of American joint committee on cancer (AJCC) staging system (tumor- node-metastases; TNM system) and consists of tumors at stages II and III. EGJ tumor definition was according to the Siwert classification that classified EGJ adenocarcinomas to three types: type I, type II and type III (4). The patients had tumor types of II or III in this classification. The exclusion criteria were: disease stages I or IV at 7th AJCC staging system, history of preceding malignancy, history of preceding RT or chemotherapy, performance status (PS) score 3 or 4 in Eastern cooperative oncology group (ECOG) scale, and coexistence of any disease that precludes oncologic treatments such as liver or kidney diseases. Ultimately, 41 patients were enrolled to the study from October, 2012 to December, 2014.
2.2. Evaluation
All patients with positive endoscopic biopsy for adenocarcinoma of EGJ or proximal of the stomach were evaluated by: complete clinical examinations, chest and abdomenopelvic computed tomography (CT) scan, and laboratory tests including complete blood count (CBC) , liver function tests (LFT), and renal function tests (RFT).
2.3. Treatment
All patients planned to undergo preoperative concurrent CRT by one of these two protocols: capecitabine 625 mg/m2 twice daily concurrent with RT or 5flourouracil (5FU) 325 mg/m2 and leucovorin 20 mg/m2 in the first four days and the last three days of RT course. Total RT dose was 45 - 50.4 grays (G) in daily fraction of 1.8 - 2 G and five days in week. RT was planned by three dimensional conformal RT (3DCRT) and radiation field was designed to cover the stomach and regional lymphatic basins. The patients evaluated every week and CBC test controlled at week of 3 - 4 of RT course for detecting treatment side effects. Four to six weeks after completion of preoperative CRT, the patients were evaluated for metastatic disease by chest and abdomenopelvic CT scan and non-metastatic cases underwent surgical resection. Then, all surgical specimens were assessed pathologically for completeness of resection (R0 or R1 resection) and pathologic response rate of tumor to preoperative treatment and the patients were followed for surgical complications such as anastomotic leakage and postoperative mortality at the first month.
2.4. Data Collection
The following data were reviewed in our database: age, gender, body mass index (BMI) ECOG PS score, pathologic classification of gastric adenocarcinoma including intestinal and diffuse types according to Lauren classification (1), location of tumor according to Siwert classification , type II and III, resectability rate (R0: microscopically complete resection - R1: microscopically incomplete resection, R2: macroscopically incomplete resection),and tumor pathologic response rate including pathologic complete response (pCR): no tumor cells in resected specimens, pathologic partial response (pPR): tumor residual cells in less than 10% of resected specimens, no pathologic response (No res): tumor residual cells in more than 10% of resected specimens.
2.5. Statistical Analysis
Nonparametric variables were presented as the median, range and categorical variables were presented as the frequency and percentages. Categorical variables were analyzed with the Chi square test and a P value less than 0.05 was considered statistically significant. Data were analyzed by statistical package for the social science (SPSS) 11.O for windows (SPSS Inc, Chicago, IL, USA).
3. Results
3.1. Patient Demographics
A total of 41 patients with clinical stage II or III of proximal gastric or EGJ adenocarcinoma were included in this study. 30 patients (73%) were males and 11 patients (27%) were females. The median age was 64 years (range 27 - 80 years). Other patient demographics and tumor characteristics are shown in Table 1.
| Variable | Total (n = 41) |
|---|---|
| Gender | |
| Male | 30 (73) |
| Female | 11 (27) |
| Age, y/O | |
| ≤ 60 | 10 (25) |
| > 60 | 31 (75) |
| ECOG-PS | |
| 0 | 1 (2.5) |
| 1 | 17 (41.5) |
| 2 | 23 (56) |
| BMI, kg/m2 | |
| 17 > | 4 (10) |
| 17 - 25 | 25 (60) |
| 26 - 30 | 8 (20) |
| 31 - 40 | 3 (7.5) |
| 40 < | 1 (2.5) |
| Lauren classification | |
| Intestinal | 18 (44) |
| Diffuse | 2 (5) |
| Missing | 21 (51) |
| Siwert classification | |
| Type II | 28 (68) |
| Type III | 13 (32) |
Abbreviations: BMI, body mass index; ECOG-PS, eastern cooperative oncology group performance status.
aValues are expressed as No. (%).
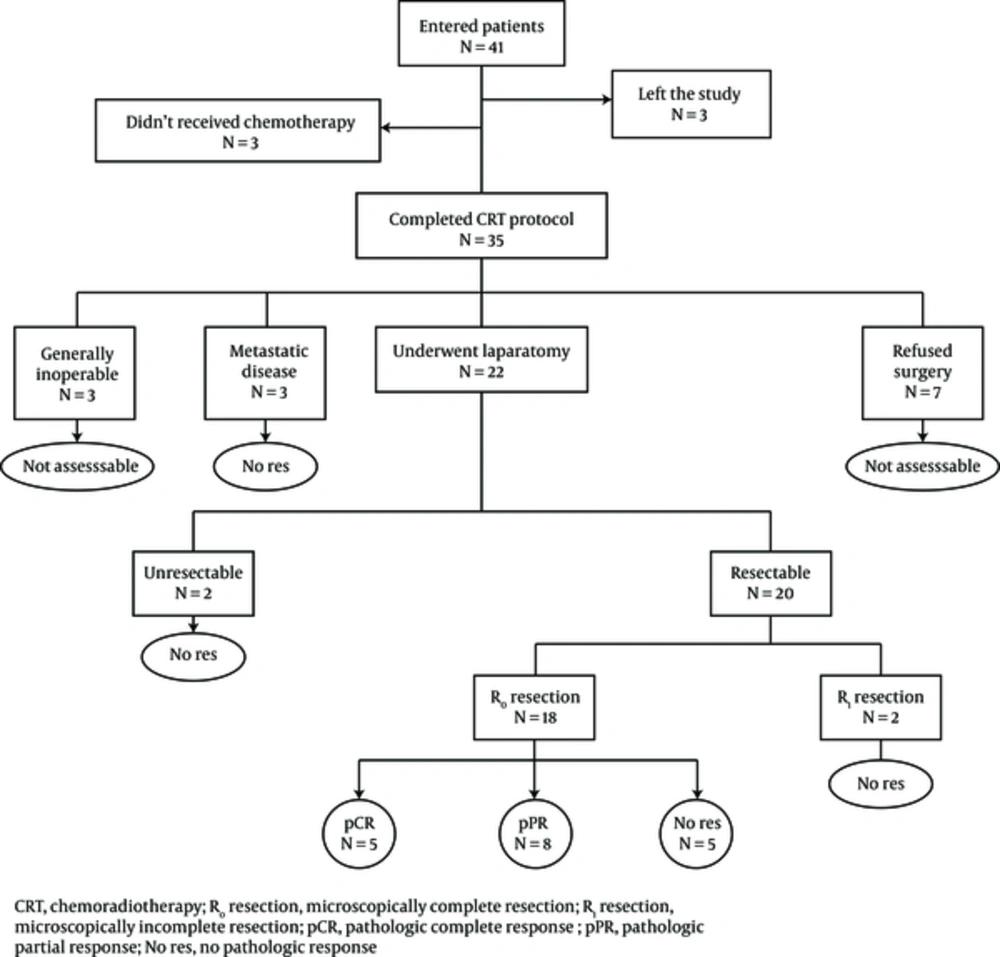
Unfortunately, primary evaluation of patients by endoscopic ultrasonography (EUS) for precise determining of pretreatment TNM staging of tumors was not accessible for our patients. However, all 41 patients were not early stage (stage I) or advanced stage (stage IV) absolutely on the basis of clinical evaluations, such as CT scan and endoscopic findings. Of 41 patients, 3 patients left the study, 3 patients refused concurrent chemotherapy with RT, and the other 35 patients completed the preoperative CRT protocol without any mortality.
3.2. Treatment Results
Patient treatment and complication details were listed in Table 2. The range of total RT dose was 43.2 - 50.4 G and the median dose was 46 G. Out of 35 patients who completed the preoperative CRT, 24 patients (68%) received 5FU + leucovorin and 11 patients (32%) received capecitabine concurrent with RT. 14 patients (40%) had no clinical treatment related toxicity and in others, the most common toxicity was anorexia in 12 patients (34%) and nausea in 7 patients (20%). We observed no World health organization (WHO) grade III or higher clinical toxicity in our patients. The most common hematologic toxicity in CBC in week 3 - 4 of CRT was leukopenia in 12 patients (34%) and 16 patients (46%) had normal CBC. Only one grade III neutropenia without any fever was observed in our patients. Of the 35 above mentioned patients, 7 patients (20%) were not satisfied with surgery and 3 patients (8.5%) were considered inoperable due to general condition. Out of 25 remained assessable patients, 3 patients (12%) had metastatic disease in preoperative evaluations due to progressive disease; therefore, their tumors were not responsive to neoadjuvant CRT (No res) and 22 of 25 assessable patients (88%) underwent surgery. The median interval between completion of CRT and surgery was 35 days (range 11 - 78 days). From these, 2 patients (9%) had unresectable tumors due to extensive adhesions during surgery (No res), 2 cases (9%) had incomplete (R1) resection (No res) and 18 cases (82%) had complete resection (R0). From 18 patients with R0 resected tumors, 5 patients (20% of assessable patients) had pCR in pathologic evaluations of resected specimens, 8 patients (32% of assessable patients) had pPR and 5 patients (20% of assessable patients) had No res. Indeed, from 25 assessable patients, we observed pCR in 20%, pPR in 32% and No res in 48%. Final results and complications of treatment are shown in Table 3.
| Variable | Total (n = 35) |
|---|---|
| Chemotherapy regimen | |
| 5FU + Leucovorin | 24 (68) |
| Capecitabine | 11 (32) |
| RT dose, G | |
| 43.2 | 1 (3) |
| 45 | 11 (31.5) |
| 46 | 11 (31.5) |
| 50.4 | 12 (34) |
| Clinical side effects | 12 (34) |
| Anorexia | |
| Nausea | 7 (20) |
| Vomiting | 5 (14) |
| Constipation | 3 (9) |
| Vertigo | 2 (6) |
| Diarrhea | 1 (3) |
| No side effect | 14 (40) |
| Hematologic toxicities | |
| Leucopenia | 12 (34) |
| Thrombocytopenia | 1 (3) |
| Anemia | 1 (3) |
| Missing | 5 (14) |
| No toxicity | 16 (46) |
Abbreviations: G, grays; RT, radiation therapy; 5FU, 5-fluorouracil.
aValues are expressed as No. (%).
| Variable | Total (n = 25) |
|---|---|
| Metastatic disease | 3 (12) |
| Unresectable tumors | 2 (8) |
| R1 resection | 2 (8) |
| R0 resection | 18 (72) |
| pCR | 5 (20) |
| pPR | 8 (32) |
| No res | 5 (20) |
| Surgical complication | |
| Anastomotic stenosis | 1 (5) |
| Death after surgery | 1 (5) |
| No complication | 18 (90) |
Abbreviations: No res, no pathologic response; pCR, pathologic complete response; pPR, pathologic partial response; R0 resection, microscopically complete resection; R1 resection, microscopically incomplete resection.
aValues are expressed as No. (%).
Schematic view of this study is shown in Figure 1. Postoperative complications were observed in only two patients. Stenosis and obstruction at surgical anastomosis in one and death at the first month after surgery in another.
3.3. Analysis
On statistical analysis, evaluation of resectability rate and its relation with some factors such as age, gender, BMI, etc. were impossible due to high rate of complete (R0) resection versus incomplete (R1) resection (18 versus 2 in 20 resected tumors). Evaluation of tumor pathologic response rate to preoperative CRT and effects of clinicopathologic factors on this response are shown in Table 4. On univariate analyses, only the male gender associated with a significant increased rate of pathologic response to preoperative CRT (P value = 0.034) and effects of the other factors were not statistically significant.
| Variable | pCR (n = 5) | pPR (n = 8) | No Res (n = 12) | Chi Square P Value | |
|---|---|---|---|---|---|
| Gender | 6.771a | 0.034 | |||
| Male | 2 | 8 | 6 | ||
| Female | 3 | 0 | 6 | ||
| Age, y/O | 2.973a | 0.226 | |||
| ≤ 60 | 2 | 1 | 6 | ||
| > 60 | 3 | 7 | 6 | ||
| ECOG-PS | 0.661a | 0.719 | |||
| 1 | 2 | 5 | 6 | ||
| 2 | 3 | 3 | 6 | ||
| BMI | 0 | 1 | 1 | 1.487a | 0.829 |
| < 17 | |||||
| 17 - 25 | 2 | 4 | 7 | ||
| > 25 | 3 | 3 | 4 | ||
| Lauren classification | 7.165a | 0.127 | |||
| Intestinal | 1 | 6 | 8 | ||
| Diffuse | 1 | 1 | 1 | ||
| Missing | 3 | 1 | 0 | ||
| Signet ring | 0 | 0 | 3 | ||
| Siwert classification | 1.997a | 0.369 | |||
| Type II | 4 | 6 | 6 | ||
| Type III | 1 | 2 | 6 | ||
| RT dose, G | 0.731a | 0.694 | |||
| < 50.4 | 4 | 5 | 7 | ||
| 50.4 | 1 | 3 | 5 | ||
| Chemotherapy regimen | 4.385a | 0.625 | |||
| 5Fu + Leucovorin | 3 | 7 | 7 | ||
| capecitabine | 2 | 1 | 5 | ||
| CRT-surgery interval (n = 22) | 6.413a | 0.17 | |||
| < 4 wks | 1 | 3 | 2 | ||
| 4 - 6 wks | 2 | 5 | 2 | ||
| > 6 wks | 2 | 0 | 5 |
Abbreviations: BMI, body mass index; ECOG-PS, eastern cooperative oncology group performance status; No res, no pathologic response; pCR, pathologic complete response; pPR, pathologic partial response; R0 resection, microscopically complete resection; R1 resection, microscopically incomplete resection; Wks, weeks.
4. Discussion
In the present study, we evaluated the effects of preoperative concurrent CRT with fluoropyrimidine based chemotherapy and other clinicopathologic factors in tumor resectability and pathologic response rates of locally advanced proximal gastric and EGJ adenocarcinomas. Few studies have evaluated preoperative chemotherapy or CRT in patients with gastric or EGJ adenocarcinomas. For example, Ajani et al. (5) treated 33 patients with gastric cancer by 5FU + leucovorin + cisplatin induction chemotherapy regimen followed by concurrent CRT with infusional 5FU and RT dose of 45 G in 25 daily fraction and 5 days in weeks. Finally, 28 patients underwent gastrectomy and lymphadenectomy. Pathologic responses including pCR and pPR were observed in 64% of all patients who underwent surgery and the median survival was significantly higher (64 months) in these patients in comparison with others who had not tumor response (13 months). Stahl et al. (6) compared neoadjuvant chemotherapy with neoadjuvant CRT in 126 patients with locally advanced EGJ or cardiac adenocarcinoma (POET trial). 119 patients were finally assessable. There was no difference between the two groups in R0 resection rate but pCR rate was significantly higher in CRT group (15.6% versus 2%). Improvement in three years overall survival (OS) was obtained by addition of RT to chemotherapy to neoadjuvant treatment.
Ajani et al. (7) treated 41 patients with concurrent with RT in preoperative setting followed by surgery in 40 patients. R0 resection rate was observed in 78%, pCR in 20% and pPR in 15% of patients. Tumor pathologic response, R0 resection rate and postoperative TNM staging were effective factors on OS and disease free survival (DFS). Van Hagen et al. (8) compared neoadjuvant CRT followed by surgery with surgery alone in esophageal and EGJ carcinomas (CROSS trial). R0 resection, pCR and OS was significantly higher in CRT group. Ajani et al. (9) evaluated induction chemotherapy followed by concurrent CRT in preoperative setting and 5-6 weeks later, surgery was performed (RTOG 9904). R0 resection was observed in 77% and pCR in 26% and OS was higher in patients with CRT in comparison with others. Wydmansky et al. (3) treated 40 patients with gastric cancer by preoperative CRT with 5FU based regimen concurrent with RT dose of 45 G followed by surgery and adjuvant chemotherapy. They observed no treatment related toxicity of WHO grade 4. Preoperative CRT was associated with higher 2 years OS, R0 resection and pathologic response rates and lower local recurrence. Pepek et al. (10), Kirsten Trip et al. (11), and Lowy et al. (12) also evaluated effects of preoperative CRT on gastric cancer in their studies and obtained improvement in R0 resection, pathologic response and patient survival rates by this treatment.
In our study, we evaluated preoperative CRT with fluoropyrimidine based chemotherapy because these agents have approved radiosensitizing effects (13, 14). We observed no significant difference in tumor pathologic response by administration of 5FU in comparison with capecitabine. In this trial, 41 patients enrolled and the majority of them were males like the other studies. The age range of our patients was 27 - 80 years, similar to the study by Pepek et al. (10) and the median age was 64 years similar to many of other studies that median age of their patients was 55 - 65. We found a statistically significant relation between male gender and tumor pathologic response rates to preoperative CRT as opposed to Ajani et al. (7) that gender had no effect on their results. Most of our patients had ECOG PS score of 2 as opposed to some studies (13, 14) that the majority of their patients had ECOG PS score of zero but like the others, this factor had no effect on treatment outcomes. In this trial, the patient BMI was a null factor on tumor resectability and pathologic response rates but high BMI was associated with increased postoperative complications. This finding supports the findings from a study by Valenti et al. (15). Histopathologic types of gastric adenocarcinoma on the basis of Lauren classification (intestinal or diffuse type) had no effect on tumor responses but we observed that all three patients whose disease progressed and became metastatic after preoperative CRT had signet ring tumor subtype and it may indicate more aggressive biology and resistance of this subtype to preoperative CRT. The median RT dose in different studies was 40 - 45 G and in our study was 46 G. The value of low or high RT doses have not been evaluated in clinical trials and we also did not find any relation between RT dose and tumor pathologic response. In this trial, our patients did not experience any WHO grade III or higher treatment related toxicity except one grade III neutropenia similar to Wydmanski et al. (3) in opposed with Orditura et al. (14), Roth et al. (16), Pepek et al. (10), and Trip et al. (11). We had no treatment related mortality during preoperative CRT. In postoperative complications, stenosis of surgical anastomosis had not been reported in other studies. However, we observed one patient with this complication but did not observe any complications reported in the other studies such as pneumonia, anastomotic leakage, sepsis and wound complication. The median interval between completion of preoperative CRT and surgery in this study was 35 days that was similar to Wydmanski et al. (3) but this factor had no effect on tumor pathologic response. Acquisition to tumor R0 resection and pCR by neoadjuvant treatments was the aim of different studies because these factors have resulted in increasing patient survival rates (5-10). In our trial, achieving this aim was promising and comparable with many other studies that are shown in Table 5.
| Study | Patient Number | Ro resection, % | pCR, % | pPR, % |
|---|---|---|---|---|
| MD. Anderson (Lowy et.al) | 24 | 95 | 11 | - |
| Multi | 33 | 82 | 36 | 28 |
| MD. Anderson (Ajani et.al) | 41 | 78 | 20 | 15 |
| RTOG 9904 (Ajani et.al) | 49 | 77 | 26 | - |
| Pepek et.al | 48 | 86 | 19 | - |
| Wydmanski et.al | 40 | 94 | 17.5 | 20 |
| Kirsten Trip et.al | 25 | 72 | 16 | - |
| This study | 41 | 82 | 20 | 32 |
Abbreviations: No res, no pathologic response; pCR, pathologic complete response; pPR, pathologic partial response; R0 resection, microscopically complete resection; R1 resection, microscopically incomplete resection.
This study had some limitations such as the small number of patients, unavailability to EUS for pretreatment evaluation of patients and short time of post treatment follow up.
4.1. Conclusions
Preoperative concurrent CRT with 5FU and leucovorin or capecitabine followed by surgery is a tolerable and safe treatment with low rate and manageable side effects, acceptable morbidity and mortality rates in patients with locally advanced proximal gastric and EGJ adenocarcinomas. This protocol results in promising high rate of tumor resectability and pathologic response. Further studies with more cases and longer follow up for detection of the best approach are warranted.