1. Background
Adenoid cystic carcinoma (ACC) is one of the most common malignancies of salivary glands and accounts for 25% of all malignant salivary glands neoplasms (1). ACC displays epithelial and myoepithelial differentiation, which arranges in tubular, cribriform, and solid pattern. Myoepithelial cells possess a dual epithelial and smooth muscle phenotype, which are reactivated for P63 and smooth muscle actin (SMA) (2). Maspin (mammary serpine protease inhibitor), as an angiogenic factor and tumor suppressor, is one of the most important products of mammary myoepithelial cells. Moreover, the modulation of matrix metalloprotainases (MMPs) and extracellular matrix (ECM) by mammary myoepithelial cells have been demonstrated (3-5). Most of the studies focusing on salivary gland tumors assessed diagnostic aspects of these markers, specifically P63 in differential diagnosis of tumors, but their utility as prognostic markers in malignant salivary gland tumors, including ACC is still elusive.
2. Objectives
The aim of present study was to evaluate the expression of P63, maspin, and matrix metalloprotainse 2 (MMP-2) in ACC to determine the association with clinicopathologic variables and mutual correlation. To confirm the myoepithelial differentiation of tumor cells, SMA staining was also carried out.
3. Methods
3.1. Study Population
The present study included 32 cases with ACC diagnosed at the pathology department, Shahid Beheshti University of Medical Sciences, Tehran, Iran. This study was approved by Ethics committee of Shahid Beheshti University of Medical Sciences.
Clinicopathologic information on each case including sex, age, tumor location, tumor size, histologic grade, lymph node metastasis, and perineural invasion were obtained from the medical records and slides.
Based on the World Health Organization (WHO) classification, 3 histologic grades were determined: grade 1, tumors with tubular and cribriform areas without solid components; grade 2, cribriform tumors that were either pure or mixed with < 30% solid areas; and grade 3, tumors with > 30% solid pattern (1).
3.2. Immunohistochemistry (IHC)
The sections (3-µm thickness) of formalin- fixed paraffin- embedded blocks were mounted on silane- coated slides (silanized S 3003; Dako, Copenhagen, Denmark), dewaxed in xylene, rehydrated in graded ethanol, and, then, treated with 3% hydrogen peroxide. Antigen retrieval was done in l0 mM citrate solution (PH 6.0) at 750 Watts in a microwave oven for l0 minutes. Sections were, then, incubated with monoclonal mouse anti-bodies under the conditions shown in Table 1, followed by treatment with Dako Envision TM. Finally, the sections were incubated with 3, 3 diaminobenzidine (K3468; DAKO) for 2 to 5 minutes at room temperature and counterstained with Mayer’s hematoxylin. Human breast tissue, ulcerative colitis, normal salivary gland, and bowel wall were utilized as positive controls for P63, MMP-2, maspin, and SMA, respectively. The primary anti-body was omitted as a negative control.
| Anti-Body | Source | Dilution | Incubation Time |
|---|---|---|---|
| Anti P63 | Dako, Denmark | Ready to use | 30 min |
| Anti maspin | Novocastra, UK | 1:50 | 30 min |
| Anti MMP-2 | Novovcastra, UK | 1:60 | 4°C,over night |
| Anti SMA | Novocastra, UK | 1:50 | 30 min |
3.3. Evaluation of IHC
P63 immunostaining was graded semi-quantitatively with negative being less than 10% of cells staining. Positive nuclear staining was graded as follows: 10% to 25% of tumor cells staining were weakly positive; 26% to 75% of tumor cells staining were moderately positive, and 76% to 100% of tumor cells staining were highly positive. The staining intensity was classified as: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining (6).
Maspin immunoreaction was ranked in to 3 groups based on the percentage of positive tumor cells: high (≥ 50% cells stained); intermediate (20% - 49% cells stained), or low (up to 20% cells stained) (7). Staining intensity was evaluated similar to P63.
MMP-2 expression was determined, using a semi-quantitative score from 0 to 6 according to the percentage of positive tumor cells (0 - 3) and their staining intensity (0 - 3). Finally, the two scores were multiplied, providing the final score: 0 to 1, negative (-); 2 to 3 (+); and ≥ 4 (++) (1).
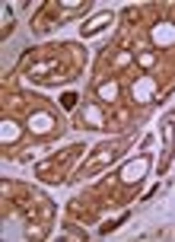
The identification of P63 positive cells as myoepithelial cell was confirmed by SMA immunoexpression.
3.4. Statistical Analysis
The data were stored and analyzed with SPSS 18 software package (SPSS, Inc, Chicago, IL, USA). Pearson Chi-square, Fisher’s exact test, Mann-Whitney, kruskal Wallis, and t test were used to assess association between P63, maspin, and MMP-2 expression and clinicopathologic variables. The analysis of correlation between P63, maspin, and MMP-2 was performed, using Spearman correlation coefficient. The level of statistical significance of all tests was set as 0.05.
4. Results
Thirty-two patients with ACC (20 females and 12 males) were studied (Table 2). The average age was 49.96 ± 13.17 years, ranging from 28 to 80 years. Of 32 cases, perineural invasion and lymph node metastasis were seen in 18 (56.3%) and 4 (12.5%) cases, respectively. Tumor size ranged from 0.8 cm to 8 cm (mean 4.03 ± 2.02).
| Characteristics | Total No of Patients (%) |
|---|---|
| Patients | 32 |
| Age, y | |
| Mean (SD) | 49.69 (13.17) |
| Range | 28 - 80 |
| Gender | |
| Female | 20 (62.5) |
| Male | 12 (37.5) |
| Location | |
| Major salivary gland | 6 (81.25) |
| Minor salivary gland | 26 (18.75) |
| Size, cm | |
| < 4 | 16 (50) |
| ≥ 4 | 16 (50) |
| Grade | |
| 1 | 10 (31.25) |
| 2 | 15 (46.87) |
| 3 | 7 (21.87) |
| Neural invasion | |
| No | 14 (56.25) |
| Yes | 18 (43.75) |
| Lymph node metastasis | |
| No | 28 (87.5) |
| Yes | 4 (12.5) |
T test showed statistically significant correlation between tumor size and lymph node metastasis (P = 0.016) .The mean size in cases with lymph node metastasis and without metastasis was 6.2 cm and 3.7 cm, respectively.
4.1. P63 Immunoexpression
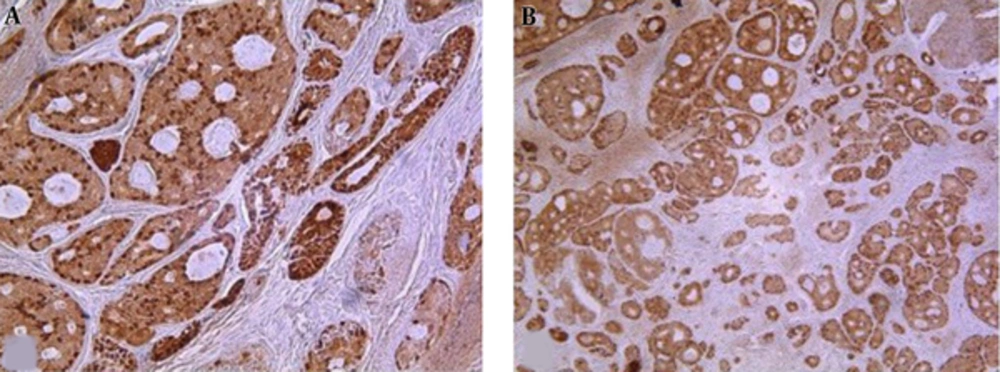
P63 was detected in 96. 8% ( 31/32) of tumors as well delineated nuclear staining mostly in abluminal cells and luminal cells that lined spaces containing mucinous material ,but those lining true lumina and displaying epithelial features were negative (Figure 1). Twenty-seven cases (87.1%) showed high expression. The remaining 4 tumors had moderate and weak P63 expression (moderate in 3 cases, weak in 1 case) (Figure 1). Strong staining intensity was also observed in 20 (64.5%) cases. All positive P63 tumor cells expressed SMA, confirming myoepitheial differentiation. P63 positive cell percentage and intensity revealed inverse significant correlation with histologic grade, respectively (P = 0.045, P = 0.025) (Table 3).
| Parameter | Percentage of Positive Cells | P Value | Intensity | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 (n) | 10% - 25% (n) | 26% - 75% (n) | 76% - 100% (n) | 0 (n) | 1 (n) | 2 (n) | 3 (n) | |||
| Grade | ||||||||||
| 1 | 0 | 0 | 0 | 10 | 0.045* | 0 | 0 | 2 | 8 | 0.025* |
| 2 | 1 | 0 | 0 | 2 | 0 | 1 | 4 | 10 | ||
| 3 | 0 | 1 | 3 | 15 | 1 | 3 | 1 | 2 | ||
| Metastasis | ||||||||||
| No | 1 | 1 | 2 | 24 | 0.805 | 1 | 4 | 7 | 16 | 0.188 |
| Yes | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 4 | ||
aP < 0.05 is significant.
P63 expression did not correlate with lymph node metastasis, perineural invasion, tumor diameter or patient’s sex and age.
4.2. Maspin Immunoexpression
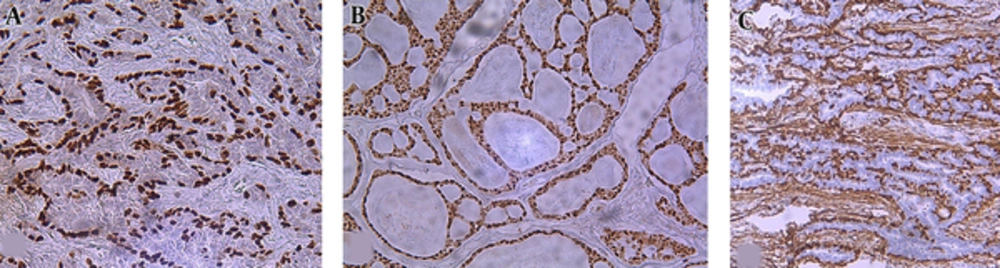
Maspin was expressed in 90.6% (29/32) of cases as cystoplasmic or nuclear- cytoplasmic positivity in luminal and abluminal cells (Figure 2). Concerning the maspin frequency of immunopositive cells varied from low to high. Most of the tumors (82.8%, 24/32) exhibited high immune staining. Furthermore, most of the cases (58.6%, 17/32) showed moderate intensity.
Twenty-six (89.7%) cases expressed maspin in both nucleus and cytoplasm and 3 (10.3%) cases in cytoplasm. Among clinicopathologic variables, histologic grade correlated inversely with maspin expression (P = 0.019) (Table 4).
| Parameter | Percentage of Positive Cells | P Value | Intensity | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 (n) | < 20% (n) | 20% - 49% (n) | > 50% (n) | 0 (n) | 1 (n) | 2 (n) | 3 (n) | |||
| Grade | ||||||||||
| 1 | 0 | 0 | 0 | 10 | 0.0 19* | 0 | 1 | 7 | 2 | 0.579 |
| 2 | 1 | 0 | 3 | 11 | 1 | 3 | 7 | 4 | ||
| 3 | 2 | 2 | 0 | 3 | 2 | 2 | 3 | 0 | ||
| Metastasis | ||||||||||
| No | 3 | 1 | 3 | 21 | 1.00 | 3 | 4 | 15 | 6 | 0.361 |
| Yes | 0 | 1 | 0 | 3 | 0 | 2 | 2 | 0 | ||
aP< 0.05 is significant.
4.3. MMP-2 Immunoexpression
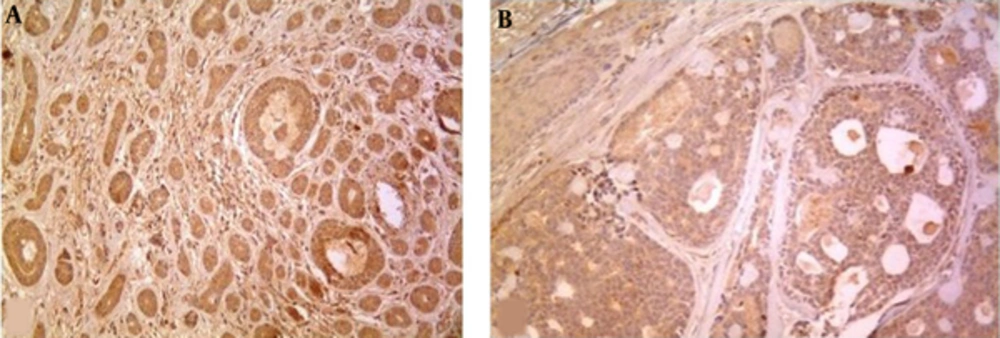
MMP-2 was expressed in 93.75% (30/32) of cases (30% of grade 1, 50% of grade 2, 20% of grade 3) as cytoplasmic staining (Figure 3).
No significant correlation was found between MMP-2 expression with clinicopathologic parameters (Table 5).
| Parameter | PERCENTAGE of Positive Cells | P Value | Intensity | P Value | Total Score | P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (n) | 1 (n) | 2 (n) | 3 (n) | 0 (n) | 1 (n) | 2 (n) | 3 (n) | 0 (n) | + (n) | ++ (n) | ||||
| Grade | ||||||||||||||
| 1 | 1 | 1 | 3 | 5 | 0.937 | 1 | 2 | 5 | 2 | 0.906 | 1 | 2 | 5 | 0.906 |
| 2 | 0 | 2 | 5 | 8 | 0 | 3 | 11 | 1 | 2 | 2 | 10 | |||
| 3 | 1 | 0 | 2 | 4 | 1 | 1 | 3 | 2 | 1 | 1 | 8 | |||
| Metastasis | ||||||||||||||
| No | 1 | 3 | 9 | 15 | 0.763 | 1 | 5 | 17 | 5 | 0.209 | 0 | 2 | 26 | 0.457 |
| Yes | 1 | 0 | 1 | 2 | 1 | 1 | 2 | 0 | 1 | 1 | 2 | |||
4.5. The Relationship Between P63, Maspin, and MMP-2 Expression in ACC
To date, this is the first study to examine the mutual correlation of p63 ,maspin, and MMP-2 expression in ACC. Spearman’s rank correlation coefficient showed significant correlation between P63 and maspin expression (r = 0.588, P < 0.001). There was no association between P63 expression and MMP-2 total score (r = 0.271, P = 0.13) as well as maspin expression with MMP-2 total score (r = 0.249, P = 0.17).
5. Discussion
ACC is the most common malignancy of minor salivary glands (8). The prognosis of ACC is unpredictable .Nevertheless, evidence reveals that histologic grade directly corresponds to prognosis (2, 9-11). Myoepithelial cells, as a component of ACC, have the potential to undergo divergent differentiation, act as a tumor suppressor, and express P63. The role of P63 in tumorigenesis is debatable. It encodes 6 different proteins with distinct and opposing function. 1 isotype, ΔNP63 may have an oncogenic role in the regulation, differentiation, and proliferation in some tumors (6, 12).
In the present study, we found positive P63 nuclear staining in 31 cases with 32 ACCS examined. The frequency and staining intensity was significantly higher in better differentiated tumors than less differentiated tumors. The study conducted by Ramer et al. was in line with this view. They demonstrated that P63 positivity can be utilized as a prognostic factor in ACC at a cut off of 35% (11). P63 expression, as a reliable indicator of histologic grade and prognosis in oral squamous cell carcinoma, breast carcinoma, and meningioma, has also been recommended (6, 13, 14). Notably, P63 is a useful marker in differentiating ACC from polymorphus low grade adenocarcinoma, basaloid squamous cell carcinoma, and neuroendocrine carcinoma (15-18).
Maspin belongs to serpin family and inhibits tumor growth by anti-angiogenic mechanism and apoptosis. However, maspin apoptotic effect appears to be tumor-specific (19). The loss of maspin has been reported with poor prognosis in breast cancers, oral squamous cell carcinoma, lung, and prostate carcinoma (7, 13). In accordance with previous studies (20, 21), the results of this study showed inverse significant association between maspin expression and histologic grade. No significant correlation was found between maspin expression and lymph node metastasis, which was similar to Ghazy et al.’s study (22), but in contrast to the findings of Schwartz et al. (23). This discrepancy may be attributed to the method of expression evaluation and the number of samples .Of note, we encountered limitation in sample collection due to incomplete data in patients’ record and lack of paraffin blocks.
One of the most important and interesting findings in the current study was significant association of p63 and maspin expression. This is supported by the previously published data in breast cancers. They suggested that p63 and maspin are the most promising markers of myoepithelial cells. Indeed, myoepithelial cells are considered to inhibit the progression of precancerous disease to invasive breast cancer (4, 13). Accordingly, we suggest p63 and maspin as the products of myoepithelial cells and important markers of biologic behavior in ACC.
MMP-2 is a significant proteolytic enzyme that degrades extracellular matrix components and facilitates invasion and metastasis. The promotion of cell growth and angiogenesis by producing MMP-2, MMP-9, and MMP- 3 have been reported in a wide variety of human cancers like squamous cell carcinoma of head and neck and breast cancers (13, 24). No relationship was observed between MMP-2 total score and clinicopathologic variables, which is in agreement with the research carried out by Zhou et al., who evaluated MMP-2 and RECK (Reversion inducing cysteine-rich protein with Kazal motif) expression in ACC (1). The modulation of MMPS expression has been indicated by myoepithelial cells through secretion of MMPS inhibitors in mammary cells and breast cancers (5, 13). In this regard, the findings of the present study may imply the presence of similar mechanism in salivary gland tumors containing myoepithelial cells. However, it requires further investigation.
Due to the 4 cm rule, malignant salivary gland tumors larger than 4 cm (T3, T4) have poor prognosis (9). They are candidates for post-operative radiotherapy. Of interest, we showed significant correlation between tumor size and lymph node metastasis, which emphasizes tumor size as a valuable prognostic factor in ACC.
In conclusion, we showed the higher frequency of P63 and maspin expression in better differentiated tumors, which suggests p63 and maspin as useful markers for predicting biologic behavior of ACC and may lead to new treatment modality in salivary gland tumors containing myoepithelial cell. Moreover, myoepithelial cell is proposed as the source of p63 and maspin expression in ACC.
Lack of association between MMP-2 expression and prognostic determinants implies the possible inhibitory role of myoepithelial cells through MMPs inhibitors secretion. The mechanism of this interrelation needs to be clarified in future studies.