1. Introduction
Adenoid cystic carcinoma (ACC) of the breast is an extremely rare neoplasm, which comprises less than 0.1% of all patients with breast cancer. This sub-type of adenocarcinoma mostly presents 3 different structural patterns: tubular, cribriform, and solid. ACCs of the breast have indolent clinical behavior and axillary node metastasis is rare. Generally, they occur in female patients with a median age of 58 to 64 years. Therefore, there are limited guidelines for treatment and diagnosis, and they are based on guidelines used for ACC of the breast in females (1-6). In the present study, we reported a rare case of ACC of the breast in a 42 year-old man.
2. Case Presentation
A 42-year-old man with no previous medical illness presented with a 1-month history of a palpable painless mass in his left breast. He denied having nipple discharge and recent weight loss. Physical examination revealed a 4 × 3 cm firm well-defined mass near the areola of his left breast with retracted nipple. An ultrasound sonography reported 2 well-defined solid-cystic masses, measured about 32 × 25 mm and 28 × 22 mm at retro region of the left breast; right breast was normal. A few small reactive nodes at both axillar regions were noted. Core needle biopsy showed the tumor cells with high N/C ratio, hyperchromatic, round to oval nuclei, inconspicuous nucleoli associated with rosette’s, few duct structures, and lumen formation mitotic activity, suggesting primary small cell carcinoma or metastasis. In immunohistochemistry (IHC) staining, tumor cells were positive for Cytokeratin, Vimentin, Ki67 (with 70% positive cancer nuclei), and CD99 was focally reactive. Tumor was negative for estrogen and progesterone receptor, HER2/neu oncogene, and thyroid transcription factor (TTF-1). IHC and cytomorphologic findings were consistent with small cell carcinoma of breast. Abdominal-pelvic ultrasound sonography and abdominal-thoracic computed tomography (CT) scans with and without IV contrast were all within normal limits and excluded metastasis. The result of Tc99m-MDP whole body bone scan, following IV injection of 15 mCi Tc99m-MDP, showed normal symmetric tracer uptake throughout the skeleton and it was negative for active skeletal metastasis. Written informed consent was obtained from the patient for the publication of this case report.
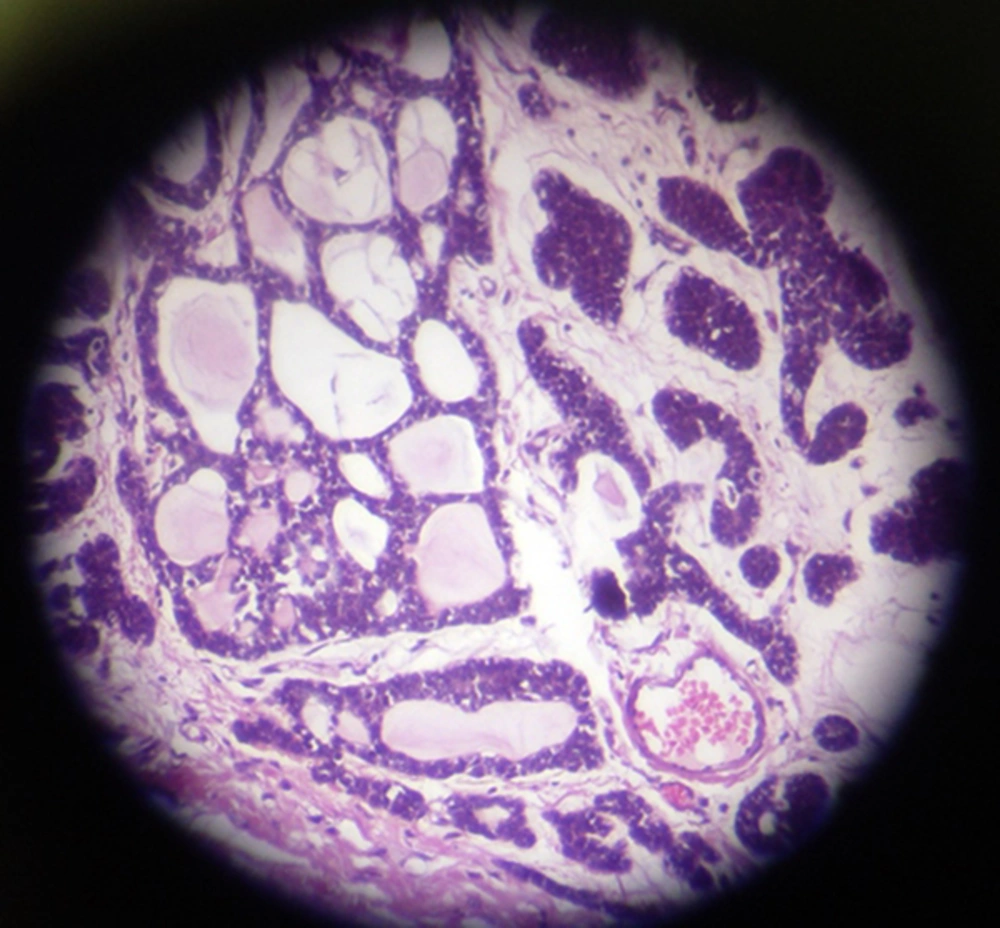
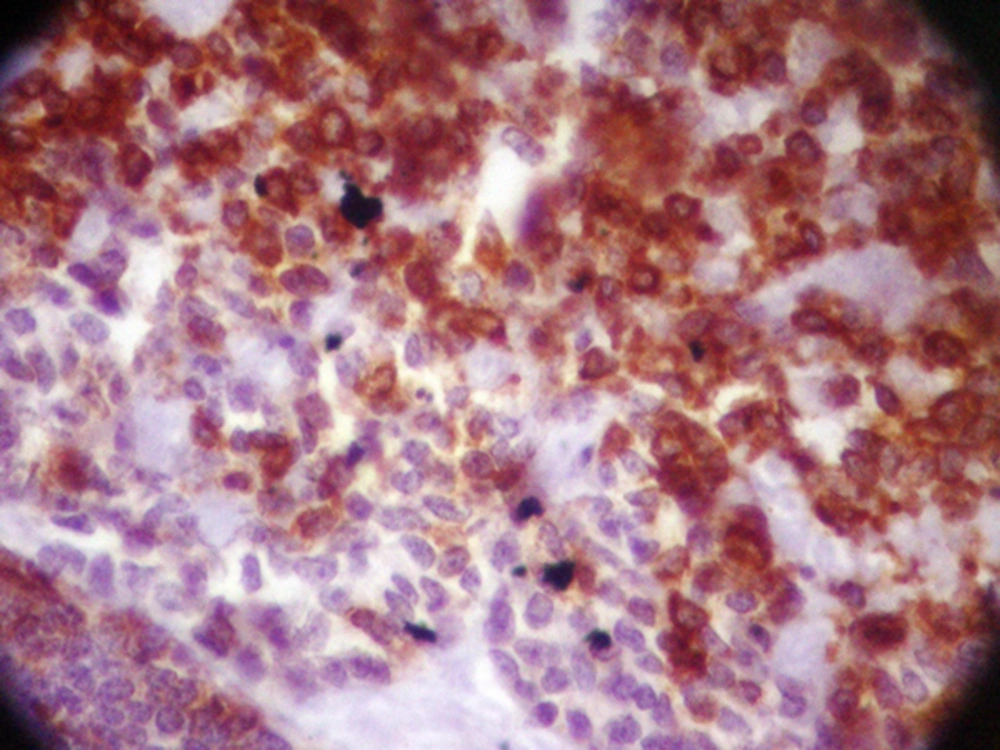
The patient underwent modified radical mastectomy (MRM) and axillary lymph node dissection (ALND) for the left breast. An intra-operative biopsy revealed solid mass measured 4 × 3.2 × 3 cm, which was away from deep margin. Breast tissue contained well-defined tumor with variable morphologic pattern, including solid sheets, trabecular, and cribriform solid pattern prominently. Tumor cells had round nuclei with coarse chromatin and scanty cytoplasm, but mitotic activity was rare. Tumor pathology was consistent with ACC (Figure 1). Some duct structures were seen too lined by lobular cells. The histopathology reported adenoid cystic carcinoma and solid variant (high grade). No vascular and perineural invasion was seen; skin and nipple were free as well as deep and lateral margin. All dissected lymph nodes were free of metastasis. The IHC staining of tumor revealed positive E-cadherin and Cytokeratin-7, and tumor was focally reactive for SMA; CD117, P63, Chromogranin, and Synaptophisin were negative (Figure 2).
Following surgery, the patient refused further treatment with chemotherapy or radiotherapy. After several follow-up sessions, there were no signs of recurrence. Now, he is alive and free of disease 12 months after mastectomy according to the results of clinical examination, bone scan, and CT scan of the patients.
3. Discussion
ACC of breast is identified by a dual cell population of luminal and basaloid cells and it is composed of both epithelial and myoepithelial components (7). They are most often considered triple negative tumors (estrogen receptor [ER], progesterone receptor [PR], and human epidermal growth factor receptor 2 [Her2] negative) (7). In the present case, the IHC of post-operation sample was negative for ER, PR, and HER2. However, in a study, ER and PR receptors were observed in 46% and 36% of ACC tumors, respectively (7). Arpino et al. found that more than half of breast ACCs expressed at least one of these receptors (2). Unlike other triple negative breast cancers (TNBC), ACCs of breast show a low proliferation activity by standard Ki-67 labeling index. Immunohistochemically, the luminal cells are positive for CK8/18, CK7, and CD117. The myoepithelial-basal cells show central oval nuclei and scanty cytoplasm. They commonly present laminin, fibronectin, basal lamina related proteins, and type IV collagen. On the other hand, the luminal cells present fodrin, E-cadherin, and β-catenin (7).
Due to the histological similarities between ACC of breast, which presents a cribriform/tubular pattern, invasive cribriform/tubular carcinoma, DCIS (Ductal carcinoma in situ) of cribriform type, and a benign condition termed collagenous spherulosis, it is difficult to make definite diagnosis. They could be misdiagnosed, when a pathologist is provided with insufficient tissue specimens by core needle biopsy. The differential diagnosis of the solid (basaloid) sub-type of ACC comprises small cell carcinoma (neuroendocrine carcinoma), solid papillary carcinoma, metaplastic carcinoma, and malignant lymphoma (8). As observed in the present study, the initial diagnosis by core needle biopsy was small cell carcinoma, whereas the post-operative biopsy revealed the different diagnosis.
Unlike the dual cells in ACCs, invasive cribriform/tubular carcinomas include hyper-proliferative single type of malignant cells only. Cribriform/tubular carcinomas generally express ER and PR, in contrast to ACCs, which are negative for both. Furthermore, the reactivity of c-Kit and/or p63 in breast ACCs has been reported in limited studies compared to the invasive cribriform/tubular carcinomas, which are negative for both (8). IHC shows several similarities between ACC and other triple negative breast cancers, except for androgen receptors (AR), which are absent in ACC, but positive in TNBC and DCIS (9).
In contrast to ACC of salivary glands, perineural invasion rarely occurs in ACC of breast. Khanfir et al. mentioned that only 5 out of 61 patients had perineural invasion and it was not considered a prognostic factor (8). In this study, perineural invasion was reported negative, too.
The age distribution of patients with ACC was reported 38 to 81 years (with a median age of 60 years) (4, 5, 9). They occur predominantly in post-menopausal women with the median age of late 50th or 60th decade (9). The average size of ACC is 3.0 cm (0.7 - 12.0 cm). Macroscopically, they have well-defined margins. They may contain pink, tan, or gray microcysts (9). ACC in females is a localized neoplasm and it usually presents as a painful, sub-areolar, and well-defined mass with nipple retraction (10). However, Giordano et al. found that, in males, it mostly presents as a firm, painless mass with palpable axillary nodes, nipple retraction, and ulceration (11). In the current study, the mass was painless, sub-areolar with nipple retraction.
Due to the extremely limited cases of male breast ACC, there is now standard protocol for treatment and diagnosis; surgery remains a main procedure of treatment. Lumpectomy, wide excision with or without radical radiation, or modified radical mastectomy is different methods of surgery. Axillary lymph node dissection has rarely been reported due to the low incidence of lymph node metastasis. Previously, the preferred treatment protocol for surgeons was radical mastectomy because of the late diagnosis of breast ACC in males and more advanced stages of the disease in comparison with females. Nowadays, surgeons prefer less extensive methods, as seen in our case; modified radical mastectomy is one of them (7). In cohort studies, local recurrence after mastectomy and breast conserving surgery was reported 0% and 6%, respectively (5). Lymph node involvement is rare in ACC and there is no need for extensive lymphadenectomy (7). In this study, the patient underwent ALND due to the primary misdiagnosis with SCC.
In ACC patients, adjuvant therapy is recommended and some evidences confirm the improvement of Overall Survival and Disease Free Survival (12). Systemic therapy is indicated for high grade lesion or tumors > 3 cm (13). Hormonal therapy and target therapy is not indicated in these cases due to the triple negative nature of ACC tumors (10).
The prognosis of ACC of breast is excellent; 5-year survival rate was reported 98% (14) and 100% (4) in some research studies. Longer follow-ups showed 95% (4) and 91% (4) survival rate in 10 and 15 years of follow-up. Female patients with breast ACCs show more favorable prognosis than their male peers. The 10-year survival rate for females with breast ACC has shifted from 85% to 100%, whereas, there is limited information about the prognosis of breast ACC in males. According to these data, male patients with breast ACC seem to be more aggressive than their female peers and it requires more aggressive treatment and follow-ups (7). Our patient spent more than a year in follow up without any recurrence; nevertheless, the surveillance period should be long, as ACC has a tendency to late recurrence.
3.1. Conclusions
ACC of breast is a rare malignancy with excellent prognosis and favorable tumor biology. It could be mistaken with triple negative breast cancer, DCIC, and small cell carcinoma. Modified radical mastectomy and lumpectomy are the mainstay of treatment and lymph node dissection is not necessary. Adjuvant therapy could be beneficial, especially in solid type and male patients. Follow-up after surgery remains prudent, despite the excellent prognosis. Nearly all local and distant recurrence occurs within 10 years of diagnosis.