1. Background
Breast cancer is one of the most prevalent cancers among women, and has been reported to be the leading cause of death from cancer in women worldwide, with over 1.3 million new cases annually (1). Invasion and metastasis are considered of the main biological characteristics of malignant tumors and significant causes of mortality in breast cancer patients (2). Metastasis and tumor growth are dependent on the development of vascular system and angiogenesis (3). Among the angiogenic factors, vascular endothelial growth factor (VEGF) and VEGF receptors (VEGFR) are considered to be the most important angiogenic factors for tumor growth (4). It is of great necessity to detect the molecular mechanisms involved in spreading breast tumor and the mechanism involved in its transformation into a malignant tumor in order to develop efficient therapeutic approaches for the treatment of breast cancer. In recent decades, some molecular investigations have demonstrated that a group of non-coding RNAs (microRNAs) contribute significantly to the onset and progression of breast cancer. Accordingly, these compounds known as biomarkers can be used for cancer diagnosis, prognosis and treatment (5, 6).
MiRNAs are a large class of evolutionarily conserved genes, and are approximately 19 - 25 nucleotides in length. These molecular structures can suppress the expression of one or more genes after transcription through binding to the untranslated regions (3'UTRs) of mRNA and decomposing mRNA or inhibiting their translation (2, 7). MiRNAs can serve as oncogene or tumor-suppressing genes and play significant roles in many cellular processes, including cell proliferation, angiogenesis, migration and apoptosis (8, 9).
MiR-126 are specifically found in the endothelial cells of capillaries and blood vessels (10, 11). Studies have demonstrated that patterns of miR-126 expression are different between breast cancer tumor tissues and healthy tissues such that in tumor tissues, miR-126 expression is considerably decreased. The difference in expression patterns results in variations in miR-126 function in the tumor tissues of patients with breast cancer (12, 13). VEGF-A has been reported to be one of the targets of miR-126 (13). VEGF-A belongs to the family VEGF, and causes proliferation and migration of vascular endothelial cells. Besides that, VEGF-A is considered to contribute significantly to metastasis and tumor growth through increasing angiogenesis and lymphangiogenesis (14, 15). Zhu et al. (2011) demonstrated that decreased expression of miR-126 in breast tumor cells resulted in increase in VEGF-A and activation of VEGF/PI3K/AKT pathway, as well as increase in angiogenesis, metastasis and induction of tumor growth (13).
In addition, the expression of miR-296 increases with progression of cancer and increase in VEGF levels (16). MiR-296 causes the induction of tumor angiogenesis through setting its downstream targets (16, 17). A study by Wurdinger et al. (2008) showed that miR-296 resulted in increased cells response to angiogenic factors of tumor tissue through targeting hepatocyte growth factor-regulated tyrosine kinase substrate (HGS), binding to HGS 3'UTR, and suppressing HGS expression. HGS gene is one of the regulatory factors of angiogenesis pathway in vascular endothelial cells; it causes a decline in angiogenesis of cancerous tumors and prevents tumor growth by down regulating angiogenic growth factor receptors, such as VEGFR2 and PDGFRB (16). Therefore, miR-296 causes increased growth factor receptors through suppressing HGS gene, which is associated with increased tumor angiogenesis.
In recent years, exercise has attracted the attention of researchers as a non-pharmacological intervention for the treatment of breast cancer and alleviation of its associated complications. Evidence indicated that moderate regular physical activity caused a reduction in the risk of breast cancer deaths (18-20). According to American college of sports medicine (ACSM), regular exercise during and after course of treatment is associated with improved physical activity, quality of life and decreased cancer-related fatigue (21). Studies have demonstrated that exercise can lead to decreased risk factors of breast cancer, including decreased sex hormones (estrogen and androgen), decreased levels of insulin and glucose, and variations in adipocytokines (increased adiponectin, decreased leptin, and decreased inflammation), as well as decreased adiposity (22). Therefore, associations of sports medicine recommend 150-min moderate-intensity exercise a week for patients with cancer (23).
Although many studies reported that regular exercise affects as an adjunct therapy to control and management of breast cancer, the mechanisms underlying this relationship at the molecular level have not been identified completely (24). The present study is performed to clarify the effect of exercise on the molecular mechanisms involved in the tumor angiogenesis.
2. Objectives
In this study, we investigated the effect of endurance training on gene expression of miR-126, miR-296, HGS and protein expression of VEGF-A, in the tumor tissues of breast cancer bearing mice
3. Methods
3.1. Animals
In the present study, twelve laboratory female BALB/c mice, weighing 19 ± 1.5 g, were purchased from the Pasteur Institute of Iran. The animals were maintained under standard laboratory conditions (23 - 25°C temperature, 40% - 45% humidity, and 12-h light-12-h dark cycle) with free access to the standard food for laboratory animals and water. All ethical principles of research involving laboratory animals approved by the Tehran University of Medical Sciences were meticulously observed.
3.2. Cell Culture
MC4-L2 cells were purchased from the University of Buenos Aires, Argentina. Then, the cell line MC4-L2 were cultured in T75 flask in DMEM/F-12 medium containing 15 mM HEPES, glutamine, 100 μg/mL penicillin, 100 μg/mL streptomycin and 10% FBS. After filling 90% of the flask with the cells, the supernatant in the medium was decanted. The cells were detached from the bottom of flask by using 0.025% trypsin, and after the process of rinsing with PBS and enzyme neutralization using 10% FBS, all the flask contents were emptied into Falcon tubes and centrifuged at 1200 rpm for 3 to 5 minutes. The supernatant was decanted and the cell plate dissolved in the medium containing 10% FBS. After that, trypan blue and hemocytometer were used to determine cell viability and cell count, respectively.
3.3. Tumor Formation
After preparation of cell suspension, one million suspended cells in PBS buffer were subcutaneously injected into the right side of the mice. Ten days after development of tumor, the mice were randomly divided into two groups of 6 each: endurance training and control group. During these ten days, the mice of the endurance training group were adapted to running on a treadmill (first, the pilot of protocols was conducted on five mice).
3.4. Endurance Training Protocol
Endurance training group performed such training for 10 weeks. Each training session involved 40-min of running on treadmill, comprising 5-min warm-up at intensity of 30% - 40% VO2max, 60-min running at 60% - 65% VO2max on treadmill, and 5-min cooling down at 30% - 40% VO2max. The protocol was performed in five days a week, for 10 weeks. On the sixth day of each week, VO2max was measured. A day in a week was assigned for rest. The control group performed no exercise, but they were placed on an immobile treadmill five sessions of 10- to 15-min a week to adapt to the environment.
3.5. Assessment of Tumor Volume
At the end of each week, tumor volume was bi-dimensionally measured using a digital caliper. The longer dimension was measured as the length of the tumor while the other dimension (at an angle of 90°) was measured as the width. Tumor volume was calculated as π/6 × width × length2, which is a standard formula for calculating tumor volume in mouse models of breast cancer.
3.6. Necropsy
Twenty-four hours after the last training session, all mice were anesthetized with intraperitoneal injection containing ketamin (90 mg/kg) + xylazine (10 mg/kg), and the tumor tissues were taken out immediately and maintained in frozen (-80°C) nitrogen until later analyses.
3.7. miRNA Expression by Real-Time-PCR
Total RNA and small RNA from tumor tissue were extracted using RNeasy Mini Kit (Qiagen, Germany), and total RNA (1 microgram) and small RNA (2 microgram) were first reversely transcribed into cDNA using miScript II RT Kit, respectively. Quantitative real-time polymerase chain reaction (PCR) (qPCR) was performed with SYBR Green RT-PCR Master Mix kit (Catalog no. 13D25, ampliqon, Denmark) in a Rotrogene 6000 system (Corbet, Germany) using the miR-126 (Catalog no. MS0005999, Qiagen, Germany) and miR-296 primers (Catalog no. MS00011613, Qiagen, Germany) set and SNORD-61 primers set (Catalog no. MS00033705, Qiagen, Germany). All samples were normalized to internal controls, and the relative expression level was calculated using the 2-ΔΔCt analysis method. Experiments were performed in duplicate samples.
3.8. mRNA Expression by Real-Time-PCR
Total RNA and small RNA from tumor tissue were extracted using RNeasy Mini Kit (Qiagen, Germany), and total RNA (1 microgram) and small RNA (2 microgram) were first reversely transcribed into cDNA using Transcriptor first strand cDNA synthesis kit (Roche, germany), respectively. Quantitative real-time PCR (qPCR) was performed with SYBR Green RT-PCR Master Mix kit (Catalog no. 13D25, ampliqon, Denmark) in a Rotrogene 6000 system (Corbet, Germany) using HGS primers set (RDG. ID: 733650, Sina gene, Iran) and GAPDH primers set (RDG. ID: 10619, Sina gene, Iran). All samples were normalized to internal controls, and the relative expression level was calculated using the 2-ΔΔCt analysis method. Experiments were performed in duplicate samples.
3.9. Western Blot Analysis
For Western blot analysis, tissue samples were centrifuged and re-suspended in ice cold RIPA lysis buffer (0.5 M Tris-HCl, pH 7.4, 1.5 M NaCl, 2.5% deoxycholic acid, 10% NP-40, 10 mM EDTA) in the presence of Protease Inhibitor Cocktail. To confirm the expression of VEGF-A, 30 μg of protein was loaded and size-fractionated on a 10% sodium dodecyl sulphate-polyacramide gel electrophoresis (SDS-PAGE) and blotted overnight onto a PVDF membrane. The membranes were blocked with TBS-T (Tris-buffered saline containing 0.3% Tween 20) and 5% nonfat dry milk for 1 h at room temperature. Western blotting was done using antibodies against VEGF-A (Catalog no. ab46154, Abcam, Cambridge, MA, USA) and β-actin (Catalog no. ab8227, Abcam, Cambridge, MA, USA) at 4ºC. The band density was normalized using the Image J software.
3.10. Data Analysis
All the data were reported as mean ± SD. Statistical comparisons were performed between the two groups using t-test. The level of significance was considered to be 0.05. The data were analyzed by statistical package for social sciences (SPSS) 19, at significance level of P < 0.05. To quantify Western-blot images and to draw charts, Graph Pad Prism was used.
4. Results
4.1. Endurance Training Decreases Tumor Growth
The median tumor volume of 112.04 mm3 was made up to 1158.0 mm3, after 10 weeks in the endurance training group (data not shown), and the tumor volume growth was 10.34 times greater than the first week in this group (P < 0.05; Table 1). At this point, the median tumor volume in the control group (non-exercise) was 108.38 mm3 and was made to 1314.9 mm3, in the 10th week (data not show), and the tumor volume growth was 12.13 times greater than the first week in this group (Table 1).
4.2. Endurance Training Protocol Mediates Its Effects Through miR-126 and miR-296
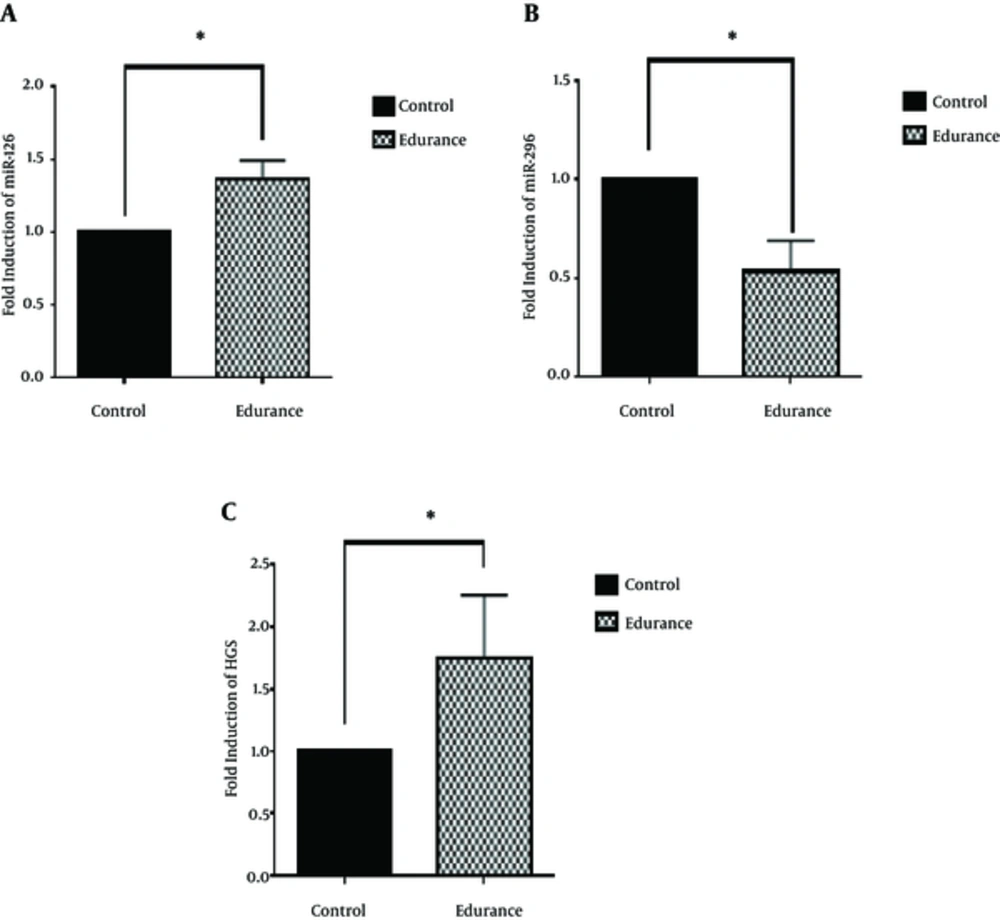
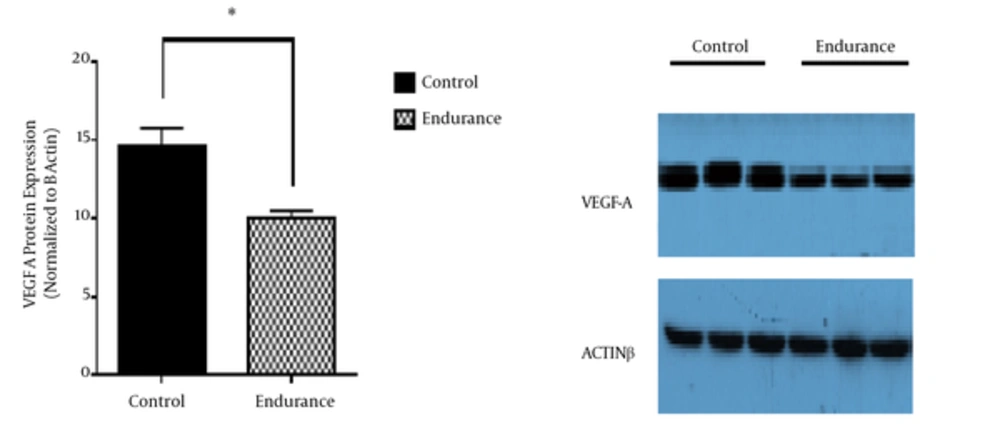
In this study, miRs expression in the tumor tissue was investigated to study the molecular mechanism of the endurance training effects on the tumor angiogenesis-induced growth. According to the findings, endurance training caused increase in miR-126 (P < 0.05; Figure 1A) and decrease in miR-296 (P < 0.05; Figure 1B) in the tumor tissue. In light of the roles of miR-126 anti-angiogenic and miR-296 proangiogenic in the angiogenesis pathway of tumor tissue, it was hypothesized that endurance training resulted in decreased tumor angiogenesis through increasing miR-126 and decreasing miR-296. To investigate this hypothesis, the expression levels of VEGF-A protein (P < 0.05; Figure 2) and HGS gene (P < 0.05; Figure 1C) of tumor tissue were measured after endurance training. The data indicated a significant decrease in the expression of VEGF-A and a considerable increase in the expression of HGS in the tumor tissue.
5. Discussion
In recent years, regular exercise has been demonstrated to considerably lead to inhibition of tumor growth and mitigation of metastatic expansion in animals and humans (25-27). In the present study, the tumor volume and tumor growth rate was decreased in the endurance training group, such that in the 10th week, tumor volume growth was 10.34 times greater than the first week, while in the control group, the corresponding rate was 12.13 times. Murphy et al. (2011) demonstrated that aerobic exercise caused a significant decrease in the tumor volume of mice with breast cancer (28). Angiogenesis is a main characteristic of cancer progression which has long been considered as a purpose in treatment. Because VEGF is considered the most important factor for angiogenesis and is directly associated with metastasis and tumor growth, it can serve as a therapeutic aim in suppressing tumor angiogenesis-induced growth (29). In this regard, Fokens et al. demonstrated that high amounts of VEGF in the primary tumor are associated with increase in metastasis and further growth of tumor in patients with breast cancer (30). Besides that, microRNAs are the regulators of gene expression at post-transcription level, which have recently attracted much attention because of their major contributions as oncogenes or tumor-suppressing genes, to the development of cancers, as well as their important roles in oncogenesis and metastasis (8).
Studies have shown that the expression levels of miR-126 are much lower in breast tumor tissue than healthy tissue (12, 13). A study by Zhu et al. demonstrated that the transduction of miR-126 into MCF-7 caused the suppression of VEGF-A. Regarding the role of VEGF-A in inducing the VEGF/PI3K/AKT signaling pathway, it can be proposed that miR-126 is an anti-angiogenic and tumor growth-suppressing agent (13). According to the findings of the present study, the endurance training caused a 37% increase in miR-126 expression and a 46% decrease in VEGF-A protein level compared to the control group. Therefore, it can be argued that the significantly increased miR-126 in the tumor tissue in the training group may explain the decreased growth of the tumor volume in the endurance training group. In this regard, Zhu et al. found that moderate-intensity running decreased tissue levels of VEGF and blood vessel density relative to sedentary control group in mice with breast cancer (31). Verma et al. investigated the association between the physical activity and the mechanisms involved in decreasing tumor volume and found that regular physical activity caused decrease in the expression of VEGF and tumor volume in mice with tumor (32).
In addition, studies have shown that miR-296 results in increase in the VEGF and plays a proangiogenic role through suppressing HGS expression and increasing the level of angiogenic growth factor receptors (VEGFR). A study by Wurdinger et al. demonstrated that miR-296 suppression by antagomiRs caused increased expression of HGS and subsequently decreased angiogenesis in tumor tissue (16). In the present study, the endurance training caused a 48% decrease in miR-296 expression and a 75% increase in HGS expression compared to the control group.
5.1. Conclusions
Our results demonstrate that endurance exercise caused a delay in tumor growth, in the endurance training group relative to the control group. We found that endurance training has an effective role in reduction of tumor angiogenesis and decreases expression of VEGF and functional VEGFRs in the tumor tissue by regulation of angiomiRs.