1. Background
The most common cancer diagnosed in women in the United States is breast cancer (according to estimation of American cancer society’s). Breast cancer is also the most prevalent cancer in Iranian females, accounting for 24.6% of all cancers. The mean age of the women with breast cancer is 49.6 years (1, 2). The current therapeutic approaches have many side effects. Therefore, recent studies have turned to therapeutic agents that can induce apoptosis in cancer cells because apoptosis is one of the important mechanisms in the treatment of cancer (3).
Emodin (1, 3, 8-trihydroxy-6-methylanthraquinone) is a hydroxy-anthraquinone that is found in many medicinal plants like Frangula, rhubarb, and senna tree. Previous studies have shown that emodin has diverse biological effects such as diuretic, antibacterial, immunosuppressive, vasorelaxant, and anti-inflammatory activities (4).
Apoptosis or programmed cell death is necessary for growth and development in organisms that are multicellular. Defect of apoptosis can result in abnormal development and pathogenesis. Some of the features of apoptosis are cell shrinkage, chromosomal DNA fragmentation and chromatin condensation. Pathways involved in triggering apoptosis include intrinsic pathway and extrinsic pathway. In the case of Intrinsic pathway, Bcl-2 family proteins regulate mitochondrial outer membrane permeabilization. In this pathway, releasing of mitochondrial cytochrome c occurs then caspases such as caspase 9 and executive caspases are activated. In extrinsic pathways, death receptors including Fas, TNFαR, DR3, DR4 are activated by their ligand and subsequently caspase 8 and executive caspases are activated (4, 5).
Emodin has also been reported to have apoptotic effects on different human cancer cell lines such as LNCaP prostate cancer cell lines, human chronic myeloid leukemia K562 cell lines, LOVO colorectal cancer cells, human lung adenocarcinoma A549 cells, human colon cancer HCT116 cells, human cervical cancer hela cells, etc. However, the apoptotic effect of emodin on SKBR3 cells is remained largely unknown. This is the first time that the effect of emodin is studied on SKBR3 cells. In addition, this cell line over-expresses the HER2 gene product. According to previous studies, HER2 overexpression has been suggested to associate with tumor aggressiveness, prognosis and responsiveness to hormonal therapy in patients with breast cancer (6).
Recently emodin has attracted special interest. In vitro and in vivo studies indicate that emodin has anticancer effects on different types of cancer cell lines involving inhibition of cancer cell growth and induction of apoptosis (5, 7-11).
2. Objectives
In the present study, we examined apoptotic effects of emodin on expression of apoptosis-related genes and activity of Caspase 3 activity in SKBR3 cells.
3. Methods
3.1. Chemicals and Materials
Emodin (purity > 95%, HPLC grade) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and was dissolved in Dimethyl Sulfoxide (DMSO). DMSO, Cell culture medium, Dulbecco’s modified eagle medium (DMEM), phosphate buffered saline (PBS) and Fetal Bovine serum (FBS) were obtained from Gibco Laboratories(Grand Island, NY). MTT powder (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide) was purchased from Sigma (St. Louis, MO, USA). The human breast carcinoma SKBR3 cells were purchased from Pastor institute (Tehran, Iran). Annexin V-FITC Apoptosis Detection Kit was purchased from eBioscience (San Diego, USA). RNA extraction kit was obtained from GeneALL Biotechnology CO. LTD (Seoul, Republic of Korea). RevertAid First Strand cDNA Synthesis kit was obtained from Thermo Scientific (Hudson, NH, USA). SYBR Green Master Mix was purchased from Takara(Japan). Caspase 3 activity assay kit was obtained from Abcam (Cambridge, MA, USA).
3.2. Cell Culture
SKBR3, the human breast cancer cells, were cultured in DMEM supplemented with 10% (v/v) of fetal bovine serum (FBS), 100 units/mL penicillin and 100 μg/mL streptomycin in a T25 flask at 37°C in a humidified atmosphere of 5% CO2.
3.3. Cell Viability Assay
Cells (5000 cells/mL) were seeded into 96 well microplates and were incubated for 24 hours at 37°C. After 24 hours, the media was replaced by 100 µL freshly prepared media containing different concentrations of emodin (0, 10, 25 and 50 µM). All treated cells were incubated further for 24, 48 and 72 hours. At the end of each time point, the cells were washed with PBS and 20 µL of 5 mg/mL MTT was added to each well. The plates were incubated for another 3 hours. Then blue formazan crystals were dissolved by adding 100 μL Dimethyl Sulfoxide (DMSO) to each well and were shaked slowly for 10 minutes. Optical density (OD) was measured at 570 nm by an ELISA plate reader.
3.4. Qualitative Determination of Apoptosis Using Flow Cytometric Assay
SKBR3 cells (1 × 106) were treated with different concentrations of emodin (0, 10, 25 and 50 µM). After 48 hours incubation, cells were harvested by Trypsin, were washed with phosphate buffered saline (PBS) and were centrifuged at 1000 rpm for 4 minutes. Then, 320 μL of binding buffer was added to 2 mL micro tube for sedimentation. Next step, 5 μL of Annexin was added to the micro tube. After 5 minutes incubation in darkness, 10 μL of PI was added to the micro tube and the samples were incubated for 20 minutes at 25°C in darkness. Finally, cell analysis was done using coulter flow cytometer.
3.5. RNA Extraction and Real Time PCR
To extract total RNA, SKBR-3 cells (500,000 cells/well) were seeded in 6-well plate. After 24 hours, the cells were treated with emodin (0, 10, 25 and 50 µM) for 48 hours. Then RNA extraction was performed by kit (GeneALL kit).
The concentration and purity was determined using Nanodrop. For checking the integrity of RNA, extracted RNA was applied on 1% agarose gel to observe 18s and 28s bands. Then , 1 µg of total RNA was reverse transcribed to cDNA using the Revert Aid First Strand cDNA Synthesis kit and RevertAid Reverse Transcriptase (RT), a recombinant M-MuLV RT. Real-time quantitative PCR was cycled 40 times between 95°C/15 seconds and 60°C/1 minute using SYBR Green PCR Master Mix. Expression of Caspase 3, 8, 9, Bcl2 and Bax genes were normalized to the internal control (ABL), a housekeeping gene. For analysis of real time PCR, Pfaffl method was used. The relative expression ratio (R) of a target gene was calculated based on E and the CP (CT) deviation of an unknown sample versus a control, and expressed in comparison to a reference gene.
Real-time PCR primers sequences as following (Table 1):
| Gene | Forward Primer and Reverse Primer (5’ to 3’) |
|---|---|
| Caspase 3 | Forward: 5′-AAATACCAGTGGAGGCCGACT -3′ |
| Reverse : 5′-TCAGCATGGCACAAAGCGAC -3′ | |
| Caspase 8 | Forward :5′-ATCCTGAAAAGAGTCTGTGCCC -3′ |
| Reverse : 5′-TGTCATTACCCCACACAACTCC -3′ | |
| Caspase 9 | Forward: 5′-AACCTTACCCCAGTGGTGCTC -3′ |
| Reverse : 5′-ATCTGCATTTCCCCTCAAACTCTCA -3′ | |
| Bax (BCL2-associated X protein) | Forward: 5′-CATGGAGCTGCAGAGGATGATTG -3′ |
| Reverse : 5′-CCAGTTGAAGTTGCCGTCAGAA -3′ | |
| Bcl2 | Forward: 5′-TGATGGGATCGTTGCCTTATGC -3′ |
| Reverse : 5′-TCAGTCTACTTCCTCTGTGATGTTGTA -3′ | |
| ABL | Forward: 5′-CTTCTTGGTGCGTGAGAGTGAG -3′ |
| Reverse : 5′-GACGTAGAGCTTGCCATCAGAAG -3′ |
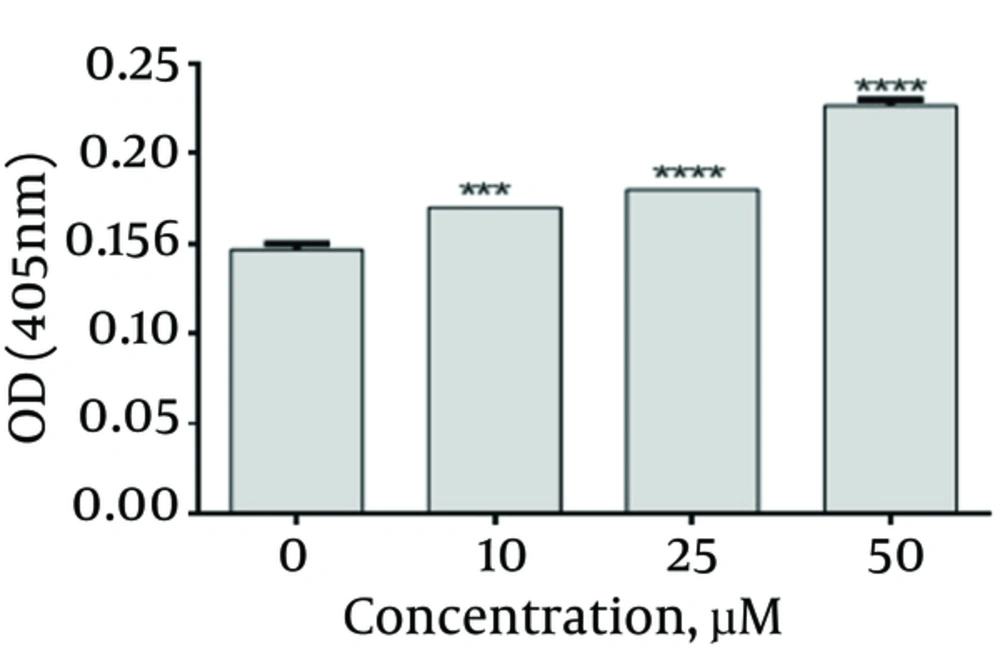
3.6. Caspase 3 Activity Assay
Caspase 3 activity was measured using a colorimetric assay. Apoptosis was induced in test samples. Then, sample proteins were extracted. After this stage, 2X reaction buffer/DTT and DEVD-pNA were added and incubation was done at 37°C for 1 - 2 hours.Optical density was measured at 405 nm.
3.7. Statistical Analysis
Data analysis was performed using SPSS v.18.One-way ANOVA (with Dunnett’s multiple range test for post hoc comparison) was performed to compare groups at different concentrations of emodin. P < 0.05 was considered statistically significant.
4. Results
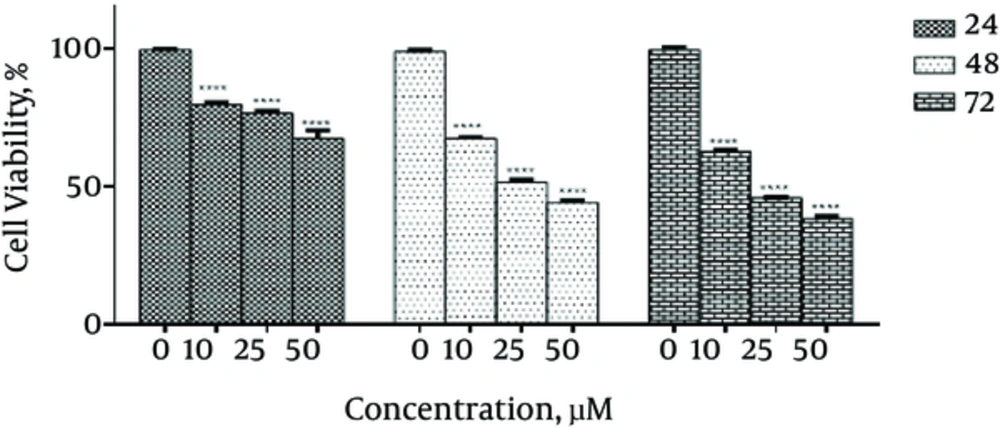
4.1. Inhibitory Effect on Cell Growth
To determine the growth inhibitory effects of emodin on SKBR3 cells and to evaluate the IC50 value, MTT assay was performed and different concentrations of emodin (0, 10, 25 and 50 µM) were used. The IC50 value was equal to 25 µM in SKBR3 cells treated with emodin after 48 hours incubation. To determine whether the inhibitory effect of emodin is time- and dose-dependent, SKBR3 cells were treated with different concentrations (0, 10, 25, 50 µM) of emodin for 24, 48 and 72 hours. The data showed that the growth inhibitory effects of emodin on SKBR3 cells are both time-and dose-dependent (Figure 1).
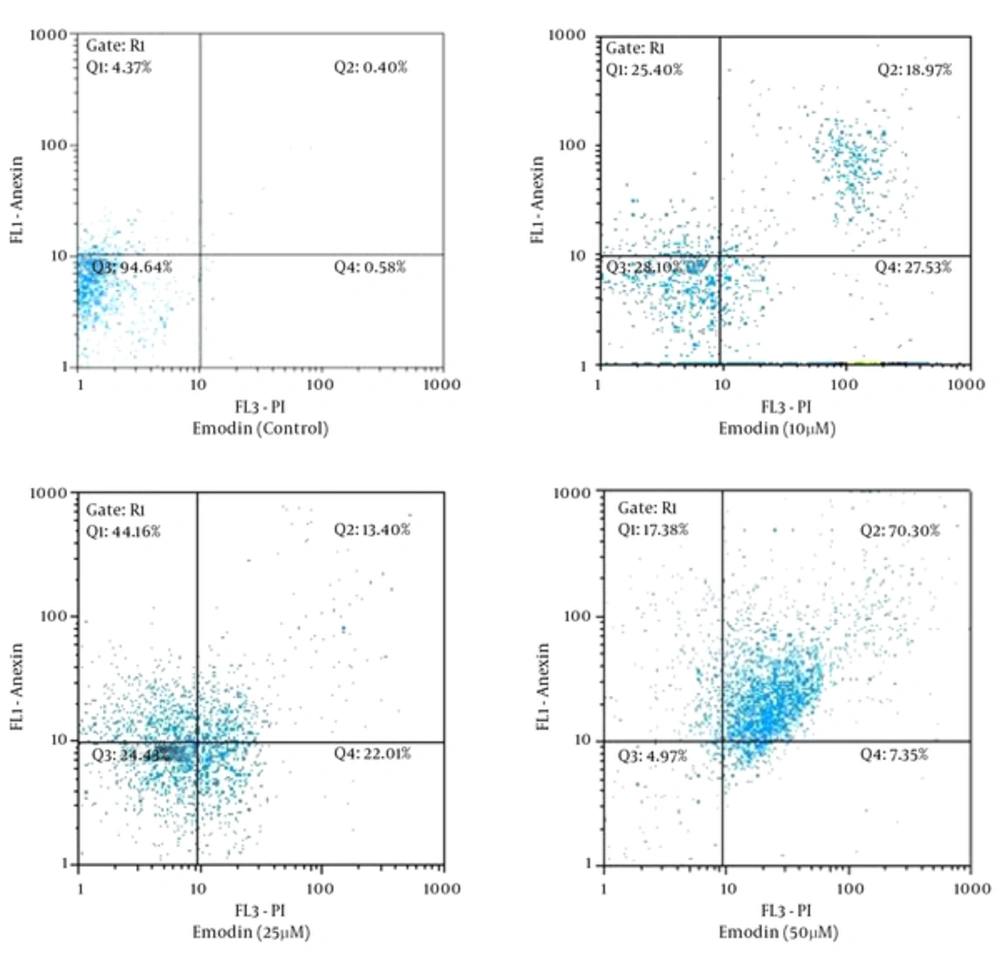
4.2. Emodin-Induced Apoptosis of SKBR3 Cells
To verify emodin-induced apoptosis of SKBR3 cells, cells were stained with annexin V-FITC and PI, followed by flow cytometry. Results are presented in Figure 2. During the emodin-induced apoptosis, normally flat cells become round in shape and detach from the extracellular matrix. These results suggest that emodin induces significant apoptosis of SKBR3 cells in a dose-dependent manner.
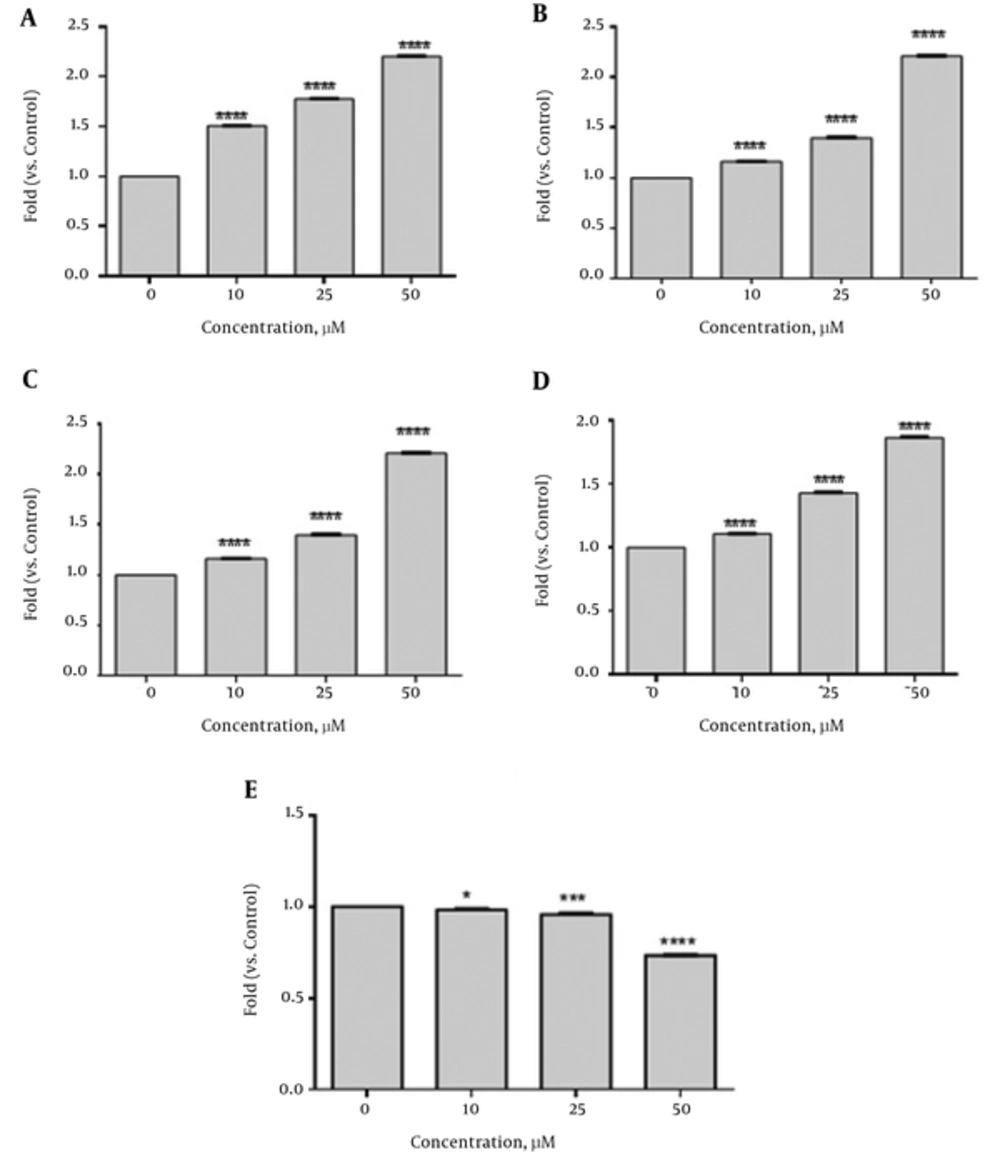
4.3. Emodin Increased the Expression of Caspase 3, 8, 9 and Bax Genes and Decreased the Expression of Bcl2 Gene
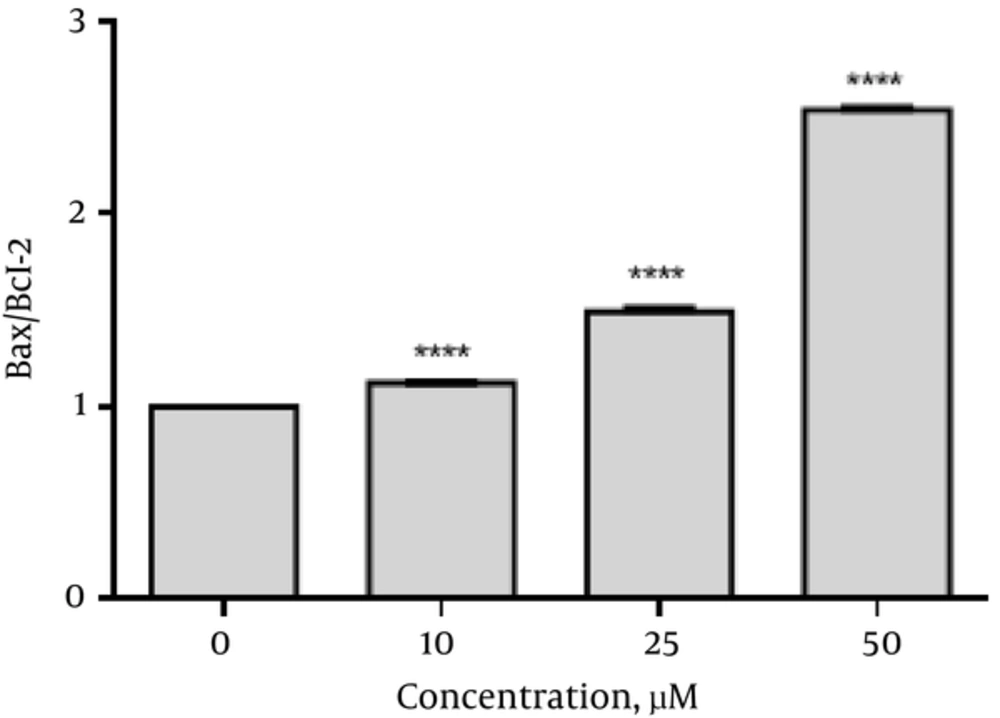
To determine the apoptotic effects of emodin on SKBR3 cells after emodin treatment (0, 10, 25, and 50 µM) for 48 hours, the mRNA levels of Caspase 3, 8, 9, Bax and Bcl2 were analyzed. As shown in Figure 3 emodin treatment (48 hours) significantly increased Caspase 3 (1.5 fold at 10 µM, 1.78 fold at 25 µM and 2.2 fold at 50 µM), Caspase 8 (1.16 fold at 10 µM, 1.40 fold at 25 µM and 2.2 fold at 50 µM ), Caspase 9 (1.51 fold at 10 µM, 1.57 fold at 25 µM and 2.9 fold at 50 µM) and Bax (1.1 fold at 10 µM, 1.43 fold at 25µM and 1.87 fold at 50 µM) gene expressions. Significant reduction was seen in expression of Bcl2 as compared with those of the control (0.98 fold at 10 µM, 0.97 fold at 25 µM and 0.74 fold at 50 µM).The ratio of Bax/Bcl2 was increased (Figure 4). However, no significant changes were seen in the gene expression of ABL (reference gene) in SKBR3 cells after 48 hours of emodin treatment (Figure 3).
4.4. Emodin Increased the Activity of Caspase 3
Caspase 3 activity was assayed using caspase 3 colorimetric kit. According to Figure 5, emodin up-regulated the Caspase 3 activity. There were significant differences when compared with their corresponding control group (emodin, 0 μg/mL)
5. Discussion
Many side effects have been observed in common therapies such as chemotherapy, and radiotherapy which are the reasons for more interest toward research in the field of herbal medicine (12-14).
According to CANCER RESEARCH UK, herbal medicine is one of the most commonly used alternative therapies and conventional allopathic medicine (CAM) by those who suffer cancer. Some studies have shown that 60% of people use herbal remedies alongside other cancer treatments. Effective compounds in the treatment of cancer derives from plants, marine organisms or micro-organism. Emodin is an example of these compounds highly used in cancer studies. Previous studies have proved that emodin has anticancer activity and induces apoptosis (5, 15, 16).
In the present study, the effects of emodin on growth and proliferation of SKBR3 cell line were examined. The results confirmed that emodin has anticancer effects with concentration of 25 µM as IC50 in SKBR3 cells (Figure 1). Besides, our results reveal that inhibitory effects of emodin on tumor cells is in a time- and dose-dependent manner.
Induction of apoptosis is among major processes through which cytotoxic drugs destroy tumor cells (17). As a result, cancer therapies have focused on this strategy (18). Apoptosis is a programmed cell death which can be induced by either intrinsic or extrinsic pathway. In this study, the mRNA expression levels of Capsase3, Caspase 8, Caspase 9, Bax and Bcl2 genes which are involved in apoptosis were investigated. All gene expressions were normalized to ABL (reference gene).
The results showed that the mRNA levels of Caspase 3, 8 and 9 were increased in SKBR3 Cells after treatment with emodin for 48 hours (Figure 2). Caspases are key factors in apoptosis. Caspase 3 is an executive caspase in both intrinsic and extrinsic pathways. Caspase 8 is an initiator caspase in extrinsic and Caspase 9 is an initiator caspase in intrinsic pathway. Various studies have also come to the same results. In prostate cancer cell line, LNCaP, emodin induced apoptosis of LNCaP cells through the upregulation of caspase-3 and -9 (19). In addition , the mRNA expression of Caspase-9, -8 and -3 increased significantly after treatment with emodin for 48 hours and the up-regulation exhibited an emodin dose-dependent pattern in human cervical cancer hela cells (18). Furthermore, emodin promoted cell apoptosis of the gemcitabine-resistant SW1990 pancreatic cancer cell line through the increase of the expression levels of caspase-9 and -3 (19). In our study, emodin up-regulated Caspase 3, 8 and 9 genes expression and could prevent the growth of SKBR3 cells through both intrinsic and extrinsic apoptosis pathways whereas it just stimulated the intrinsic apoptosis pathway in some cancer cell lines such as prostate cancer cell line, LNCaP, gemcitabine-resistant SW1990 pancreatic cancer cell line, human lung adenocarcinoma A549 cells.
Bcl-2 is an anti-apoptotic activator that inhibits the releasing of cytochrome c from the mitochondria in an intrinsic pathway. Bax is an apoptosis activator and triggers the release of cytochrome c and Smac-diablo (20). Our results have indicated that emodin is able to, at the same time, increase bax (encoding the pro-apoptotic protein Bax) expression and down -regulate bcl-2 (encoding the anti-apoptotic protein Bcl-2) expression (Figure 4). These results are in line with previous studies. Emodin induced apoptosis of LNCaP cells through the alterations in expression of Bax and Bcl2 genes which had the same result as ours (21). In another study, the increased Bax concurrent with the decrease of Bcl-2 indicated that emodin induces apoptosis in human chronic myeloid leukemia K562 cell lines (22). Furthermore, emodin induced a significant increase in the Bax expression level and a marked reduction of the Bcl 2 expression level in LOVO colorectal cancer cells (23). In this study, the ratio of Bax/Bcl2 was increased (Figure 4). According to previous studies, this ratio is considered as a predictive marker for response to cancer therapies and cancer prognosis (24-26). In the current study, induction of programmed cell death was also confirmed by flow cytometry (Figure 2). The data of flow cytometry analysis was in complete agreement with the molecular research.
Based on our results, caspase 3 activity in SKBR3 cells was increased by emodin (Figure 5). This result is consistent with our real time PCR result. This is also in line with previous studies. In one study, emodin caused rapid and transient induction of caspase 3 in HL-60 promyelocytic leukemia cell line. Moreover, emodin increased the activation of caspase-3 in human chronic myelocytic leukemia K562 cell lines. In another research, emodin-induced HK-2 cell apoptosis through the activation of caspase 3 in HK2 cells (15, 22, 27).
5.1. Conclusion
In summary, emodin could induce apoptosis in SKBR3 cells through the modulation of expression of apoptosis-related genes Emodin treatment upregulated Caspase 3, 8, 9 and Bax gene expression but down-regulated the expression of Bcl2. According to our results, initially considered hypothesis is true. Both the intrinsic and the extrinsic apoptotic pathways might be involved in SKBR3 cells. However, further studies are necessary to understand the exact mechanisms of the activities of emodin.