1. Background
The rate of breast cancer has been growing and now is considered a chief danger to women’s health. Recent breast cancer therapy comprises of surgical procedure and adjuvant postoperative local or general radiotherapy, chemotherapy and hormonal therapy. Breast cancer is a group of illnesses each with distinguished histopathological features, genetic and genomic variation, and dissimilar predictive results.
The coming on personalized medicine has the potential for deep effects on breast cancer study and treatment as its goals are to detect the particular happenings that drive growth and evolution of a personage’s cancer and to detect patient particular therapeutic objectives (1). Thus, here we focus on VEGF-A and VEGFR-2 in breast cancer therapy.
The relationship between angiogenesis and tumor has been attributed to Judah Folkman, who firstly indicated that tumour increasing was directly reliant on the blood vessel network expansion (2).
Tumor angiogenesis is an important procedure for the tumor development as it confirms oxygen and nutrients source to increase cells through the expansion of new blood vessels, potentially causing cancer development and metastasis (3).
The vascular endothelial growth factor (VEGF) family is a group of important proteins intricate in the angiogenic passageway (4).
The VEGF family includes of several signal protein variations and their receptors. Between them, the VEGF-A and VEGF receptor (VEGFR) subtype2 interaction is a major interaction in angiogenesis (5).
Previous studies indicate that vascular endothelial growth factor-A (VEGF) is an important motivation of angiogenesis, VEGF is overexpressed in maximum dense tumors, and inhibition of VEGF can repress tumour progress in animal models (6-8).
VEGF-A stimulates angiogenesis through connection to its two receptor tyrosine kinases KDR/Flk-1 (VEGFR-2) and Flt-1 (VEGFR-1), expressed on endothelial cells. KDR/Flk-1 is needed mostly for mitogenic and chemotactic responses, whereas Flt-1 participates to endothelial cell morphogenesis (9).
VEGF-A is the main form that binds and signals through VEGFR-2 to improve blood vessels and preserve the vascular network (10, 11).
VEGF and its receptor are greatly expressed in a lot of tumor forms so they may illustrate potential goals for antitumour therapy (4).
Emodin, a tyrosine kinase inhibitor, is an innate anthraquinone derivative found in the roots and rhizomes of several plants. Pharmacological studies have revealed that emodin displays various biological roles, such as anti-inflammatory, antibacterial and anticancer action. Studies have confirmed that emodin prevents cell growth in some types of cancer cells (12-14).
Emodin controls genes associated with the control of cell apoptosis, oncogenesis cell proliferation, cancer cell invasion and metastasis (15).
Emodin has also been reported to have anti-angiogenic effects and anti-metastatic effects on different human cell lines such as HCT116 (human colon cancer cells), HUVECs (human umbilical vein endothelial cells), pancreatic cancer tissues and Human tongue cancer cell line (SCC-4) (9, 15, 16).
However, because the high incidence of breast cancer and also the anti-angiogenic effect of emodin on MCF-7 cells is largely unknown, this is the first time that anti-angiogenic effect of emodin is studied on MCF-7 cells.
2. Objectives
The aim of this study was to investigate the effect of emodin on VEGF-A and VEGFR2 expression in MCF7 cell line.
3. Methods
3.1. Cell Culture
Emodin (purity > 95%, HPLC grade) was obtained from Santa Cruz Biotechnology (santa cruz , CA, USA). Stock solution of emodin (10.7 mg/mL) was dissolved in dimethyl sulfoxide (DMSO) and further diluted in culture medium. Human breast cancer cells MCF-7 were purchased from Pasteur Institute (Tehran, Iran) and cultured in Roswell Park Memorial Institute medium (RPMI) 1640 (Gibco laboratories , Grand Island, NY) complemented with 10 % fetal bovin serum(FBS)(Gibco Laboratories , Grand Island, NY), and 1% penicillin/streptomycin. Cultures were preserved at 37°C in a moistened incubator in an atmosphere of 5% CO2
3.2. Cell Viability Assay
The cells were trypsinized and approximately 5000 cells per well of 96 well plates were seeded for 24 hours at 37°C then treated with different concentration of emodin (0, 10, 20, 30, 40, 50 and 60 μM) for 24, 48 and 72 hours, respectively.
Each group of concentrations were seeded in 6 wells. Then, 20 μL of 5 mg/mL [3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) (Sigma, St. Louis, MO, USA) solution was added into each well and they were incubated for 4 hours at 37°C. After this, to ensure that the cells were attached to the floor of each well, 96 well plates were centrifuged at 3000 rpm for 5mins at 25°C (brake zero) and then the supernatant was discarded. To dissolve the purple-colored precipitate of formazan 100 μL Dimethyl Sulfoxide (DMSO) (Gibco Laboratories, Grand Island, NY) was added to each well and they were shaken slowly for 10 minutes. After complete dissolution, the absorbance was recorded at 570 nm by an Elisa plate reader (BioTek, Winooski, VT, USA). The Effect of emodin on growth inhibition was calculated as the percentage of inhibition in cell growth. Each assay was repeated three times, and the results were expressed as mean. Percentage of cell viability is measured as follow: [value of drug-treated group (A)/control group (A)] ×100.
3.3. Apoptosis Analysis
Apoptosis was measured by Annexin V fluorescein isothiocyanate (Annexin V FITC; eBioscience, San Diego, USA). For this purpose, 2 × 105 cells / well were seeded in 6 well plate and were incubated for 24 hours at 37°C. Cells were treated with different concentrations of emodin (0, 10, 20, 30, 40 and 50 μM). After 48hrs cells were collected and washed twice with phosphate buffer solution (PBS) (Gibco Laboratories, Grand Island, NY), re-suspended in 320 μL binding buffer containing an additional 5 μL Annexin V FITC and 5 μL propidium iodide, and incubated for 15 minutes at room temperature in darkness. Samples were tested by flow cytometry. Tests were carried out at least three times.
3.4. Quantitative Polymerase Chain Reaction (qPCR)
To extract total RNA, 8 × 105 MCF-7 were seeded in 6-well plate after 24 hours, the cells were treated with different concentrations of emodin (0, 10, 20, 30, 40 and 50 μM) for 48 hours and then RNA extraction was performed using kit (GeneALL Biotechnology CO. LTD (Seoul, Republic of Korea)). Purity and concentration of RNA extracted from each sample was measured by Nanodrop and RNA extracted was stored at -80°C for the synthesis of cDNA. Two micrograms of RNA from each sample were used to synthesize cDNA by M-MuLV Reverse Transcriptase according to the instructions (Thermo Scientific (Hudson, NH, USA)). CDNA created was used as a template for real-time PCR.
Real-time quantitative PCR was cycled 40 times between 95°C /15 seconds and 60°C /1 minute using SYBR green PCR master mix (Takara (Japan). Expression of VEGF-A and VEGFR-2 genes were normalized to the internal control (ABL), a housekeeping gene. For the analysis of real time PCR, Pfaffl method was used. The relative expression ratio (R) of a target gene based on E (Efficiency) and the CP (CT) deviation of an unknown sample versus a control was calculated, and expressed in comparison to a reference gene.
Real-time PCR primers sequences were as follow
VEGF-A:
forward: 5’-TCA GAG CGG AGA AAG CAT TTG TTT G -3’
reverse: 5’-CCG CCT CGG CTT GTC ACA T -3’
VEGFR-2:
forward: 5’-CTG TGG GTT TGC CTA GTC TTT C -3’
reverse: 5’- TGC TCA CTG CCA CTC TGA TTA T -3’
ABL:
forward: 5’-CTT CTT GGT GCG TGA GAG TGA G -3’
reverse: 5’- GAC GTA GAG CTT GCC ATC AGA AG -3’
3.5. Statistical Analyses
Data analysis was performed using SPSS v.18.One-way ANOVA (with Dunnett’s multiple range test for post hoc comparison) performed to compare groups at different concentrations of emodin. P < 0.05 was considered statistically significant.
4. Results
4.1. Effect of Emodin on Cell Viability of MCF-7 Cells
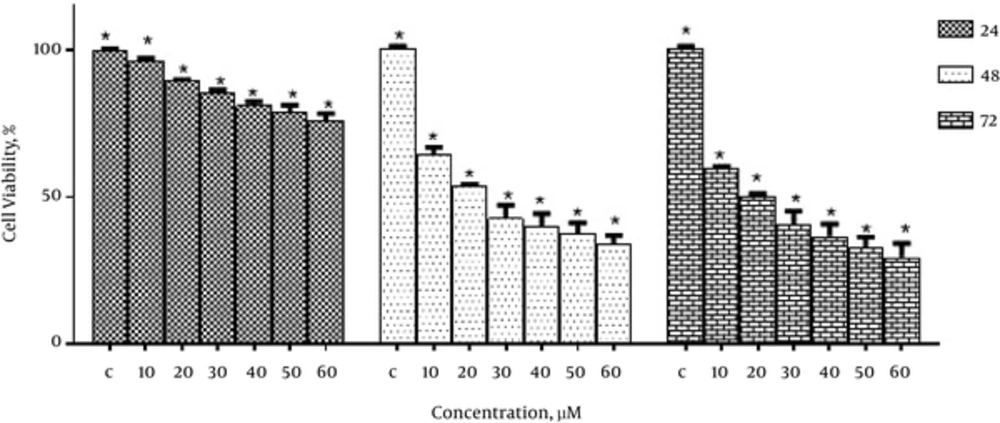
To study the inhibitory effect of emodin on the growth of MCF-7 cells, the cells were treated with different concentrations of emodin (0, 10, 20, 30, 40, 50 and 60 μM) for 24, 48 and 72 hours. Then cell viability was determined using MTT assay. As shown in Figure 1, emodin significantly reduced the growth of MCF-7 cells in a dose and time-dependent manner.
The IC50 values of emodin in MCF-7 cells was 20 μM in 48 hours and 72 hours.
4.2. The Effects of Emodin on Apoptosis
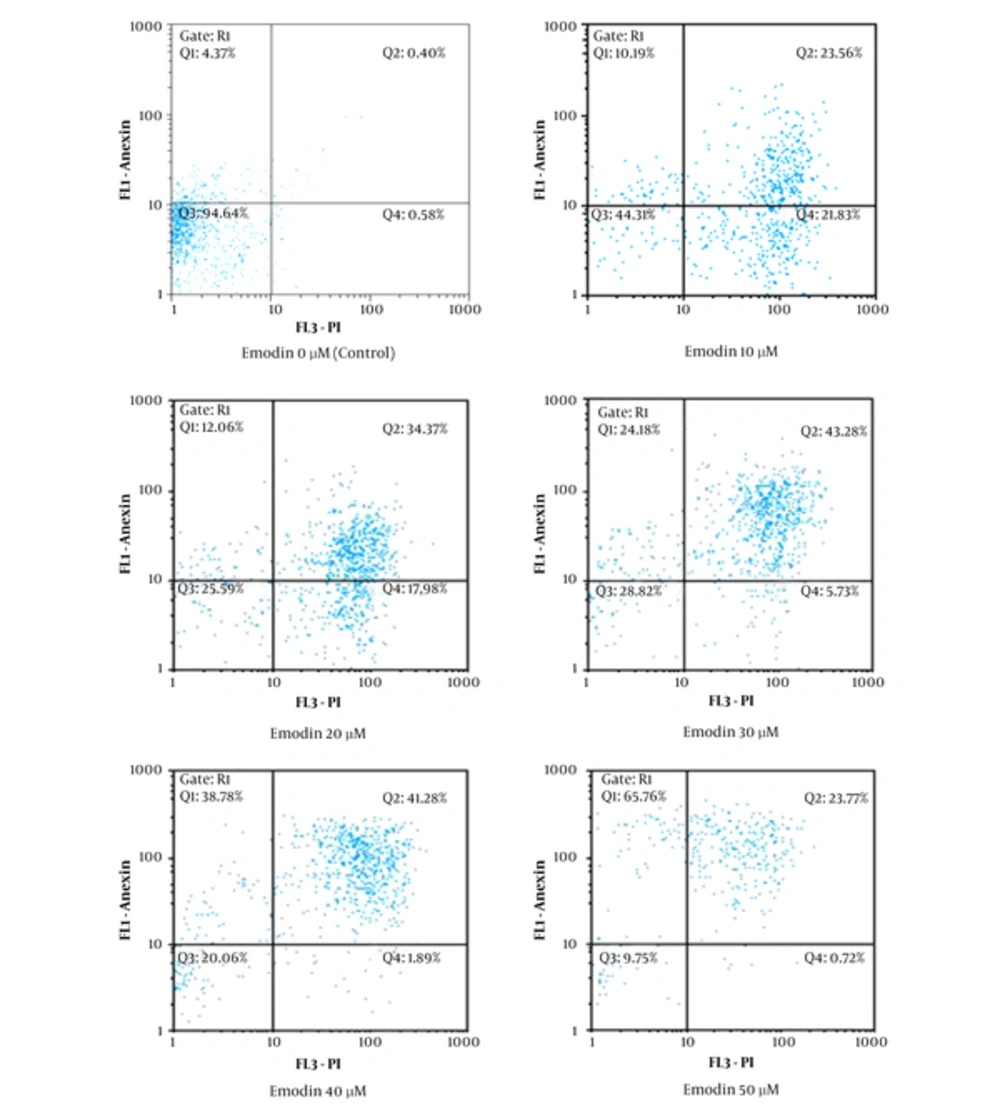
To ensure that the growth inhibitory effect of emodin on MCF-7 cells is by apoptosis, after treating MCF-7 with different concentrations of emodin (0, 10, 20, 30, 40 and 50 μM) for 48 hours, cells were analyzed by flow cytometry. As shown in Figure 2, emodin induced apoptosis in MCF-7 cells.
4.3. Emodin Suppressed VEGF-A and VEGFR-2 Expression
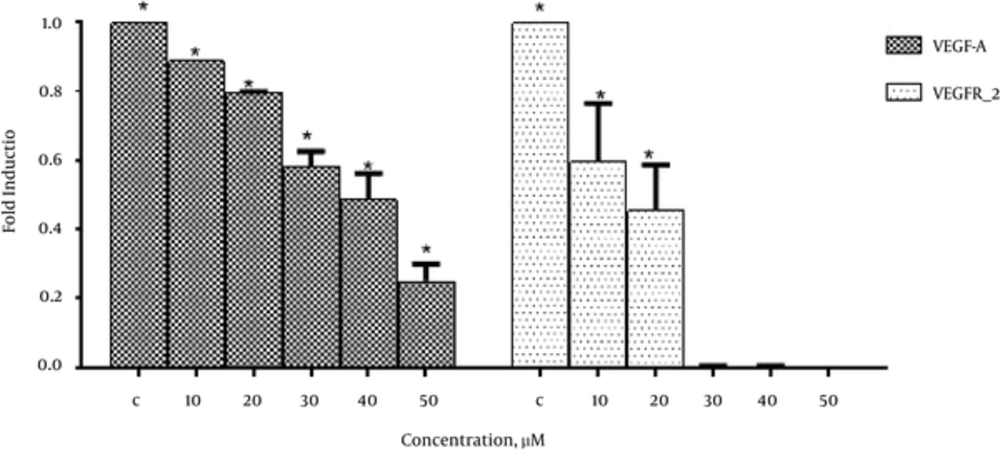
In order to investigate the effects of emodin on VEGF-A and VEGFR-2 expression real-time PCR was performed. As shown in Figure 3. Emodin significantly reduced VEGF-A and VEGFR-2 gene expression in a dose dependent manner.
5. Discussion
Breast cancer is a common malignant cancer and is one of most mortal cancers in women.
To date, chemotherapy has been considered the best treatment for breast cancer. However, the side effects and drug resistance related to chemotherapy have restricted its efficiency for the treatment of breast cancer. Thus, recent research on breast cancer management is followed by therapeutic agents that inhibit growth and induce apoptosis in cancer cells (17, 18).
Wide laboratory information supported that angiogenesis can be identified throughout the invasion, growth, and metastasis in breast cancer. Of the earliest events in angiogenesis is the secretion of several angiogenic factors from cancer cells. In the current study, VEGF has been recognized as the best significant pro-angiogenic factor (19).
Emodin, an anthraquinone derivative, can be separated from various medical plants including Rhubarb (Rheum officinale B.), Aloe barbadensis and sheet of Senna (20, 21).
A former investigation has depicted that emodin has antifungal, antibacterial, antiviral and anti-VEGF activities (22).
In this study, for the first time, the effect of emodin on angiogenesis in human breast cancer cell line MCF-7 has been studied.
In this study, we examined the effect of emodin on the cell viability of MCF-7. The results showed that emodin has anti-cancer effects with concentration of 20 μM as IC50 in MCF-7 cells (Figure 1). Data from this study showed that emodin significantly reduced the growth of MCF-7 cells in a dose and time-dependent manner.
Based on our results the anti-proliferatory effect of emodin on MCF-7 cells is by the induction of apoptosis (Figure 2).
Consistent with our results, Wing-Yan Li et al. also showed that emodin induces cytotoxic effect in MCF-7 cells through modulating expression of apoptosis related genes (17).
Many studies have shown anti-angiogenic effect of emodin on different cell lines (9, 16, 23, 24).
The current study also demonstrated that emodin significantly reduced mRNA level of VEGF-A and VEGFR-2 gene in MCF-7 cell line in a dose dependent manner whereas its inhibitory effect was much more pronounced in VEGFR-2 gene expression (Figure 3).
Down regulation of VEGF-A gene expression by emodin can probably be the result of inhibitory effect of emodin on NF-KB which is required for expression of VEGF-A gene (25).
Other possible mechanisms for down regulation of VEGF-A could be emodin induced p 53 dependent apoptosis (22).
L-Ryden et al. have shown that VEGFR-2 expression correlated with VEGF expression in breast cancer cells; in other words, these molecules are co-expressed (26). Co- expression of these angiogenic factors could perhaps be an explanation for reduced VEGFR-2 gene expression following down regulation of VEGF gene.
5.1. Conclusions
According to the results of this study, emodin possess antiangiogenic effects in MCF-7 cells and in the future we may be able to benefit from it in treatment of breast cancer.