1. Background
Cancer is known as a significant health problem in the world, especially in developed countries (1). It is recognized as the second leading cause of death worldwide and the third cause of death in Iran (2). One of the most common cancers in the world is colorectal cancer (CRC) (it is also called colon or bowel cancer) (3). CRC is a heterogeneous disease that occurs in different parts of the colon and rectum, which are parts of the gastrointestinal system. The majority of CRC develops slowly from adenomatous polyps or adenomas (4). This cancer is the third most common malignancy in the world, the second leading cause of cancer deaths in the United States, and also one of the most common cancers in the gastrointestinal cancers in Iran (5, 6). According to the Iran cancer registry program, it is estimated that after stomach cancer, CRC is the most common gastrointestinal cancer in Iran (7). Risk factors prevalence, the rate of early-stage diagnosis, and treatment recommendation for CRC are varied due to differences in incidence and mortality rates across societies and health care systems (8). Incidence and mortality of CRC in developed countries with western lifestyles such as the US and Japan have been reduced (9-11). Other countries such as Australia and France have experienced stability in the incidence rate of CRC. In Asian countries, several studies suggest that Japan is the only country to have achieved a decreased incidence and mortality in CRC (12, 13). The incidence of the CRC in recent years has increased in Iran because of dietary changes, lifestyle, changes, and reduction in physical activity and changes in diagnostic techniques. Most cases in developed countries are diagnosed after age 50. The signs and symptoms of CRC such as rectal bleeding, blood in stool, and a change in bowel habits often have been seen at a relatively late stage (14).
In the past decades, there has been considerable disparity in the level of CRC mortality between European countries. However, CRC mortality declined in the United States in both men and women (15). In Asian countries such as Iran, expect Japan and Singapore, the mortality of CRC has been increasing in the last decade (16). Moreover, the mortality rate of CRC in Iran is lower compared to European countries, but is higher than the rate in the United States (17). There is a similar linear increasing trend of mortality rate between young Iranians and Americans, which predicts the higher burden in the future worldwide (14). Lifestyle factors that include obesity, physical activity, and diet are the potential risk factors, which are associated with CRC mortality (18). In the last decade, in Asia, the 5-year survival rate of CRC was 60%, while this value in the United States was 64%. However, different studies from Iran have indicated that the 5-year survival rates of CRC were 47%, 41%, and 61%. Thus, a high proportion of survival among patients with CRC is observed. Moreover, this proportion of survival can be increased with early diagnosis of cancer (6, 19, 20).
The relative survival analysis provides survival estimates adjusted for the background risk of death at particular time points after diagnosis. In addition, the relative survival analysis is the ratio of survival, which is observed among the patients with cancer. Population cure is an extension of the relative survival concept. If relative survival curves reach a plateau after a period of follow-up, the excess mortality related to the cancer of patients is equal to the background mortality. Thus, these patients are no more likely to experience death than their counterparts in the general population. Therefore, the analysis of cure rate can provide more information about the pattern of cancer survival (21, 22).
2. Objectives
In the current study, we aimed at determining the effect of clinical, biological, and pathological characteristics of patients on the cure fraction of patients with CRC, using parametric Weibull cure model.
3. Methods
This registry-based retrospective cohort study was conducted at the Colorectal Cancer Research Center of Shiraz University of Medical Sciences, using medical records of 512 patients with CRC. Information of all patients with colon and rectum malignances were registered in Shahid Faghihi Hospital, Shiraz. They were followed-up for 8 years from January 2009 until February 2017. Based on the time protocol of the Colorectal Research Center, the patient’s information was reviewed and updated. In the first year after surgery, each patient was visited once every 3 mounts, and in the second year, once every 6 months and, finally, their information was recorded by the colorectal surgeon annually. In case of no referral, the patients or patients’ family members were contacted via phone calls at specified intervals to inquire whether the patients were still alive. In addition, the current study was extracted from an MSc thesis, which was checked and approved by the Ethics Committee of the Shahid Beheshti University of Medical Sciences (IR.SBMU.PHNS.REC.1396.91).
In the current study, death due to CRC was regarded as the failure and the time interval between the death of patients with CRC and CRC surgery was calculated as the survival time of patients with CRC. Patients who were survived after the longest event time were known as statistically cure patients.
Age at diagnosis and gender were considered as demographic information. Body mass index (BMI), history of CRC diagnosis, type of surgery, clinical characteristics, appearance of the tumor, residual tumor after chemo-radiotherapy, tumor size, lymph node ratio, American Joint Committee on Cancer (AJCC) staging, location and size of tumor, the vascular, lymphatic, and perineural invasion of tumor were identified as pathological and biological characteristics.
3.1. Statistical Analysis
Descriptive characteristics of the patients are shown as mean (± standard deviation) with interquartile range (IQR) and frequency (percentage) for continuous and categorical variables, respectively. Log-rank test was performed to assess the difference in the distribution among the levels of variables.
In survival analysis, it is usually assumed that all individuals gradually experienced the event of interest until the end of the observation period (22). In some situations, a substantial proportion of individuals do not experience the event under the study at the end of follow-up time (21). Under this circumstance, the population consists of two groups: the group of individuals who do not experience the event of interest (or long-term survivors) and the group of patients who are susceptible (or non-cured) to the disease (23). In such a situation, standard survival analysis is not a proper method because of increases in a number of censoring at the end of the follow-up period, which does not account for the possibility of long-term survivors (21). In order to confirm that a considerable number of cases do not eventually experience the event of interest until the end of the follow-up time, the Kaplan-Meier survival plot can be a useful tool to reveal whether the plot reached zero with the current amount of follow-up time; so, a significant number of cases is cured and also it appears that there is a considerable cure fraction in dataset (24). In the case of high cure fraction, Cox regression model will lead to bias estimates. Cure models allow us to build a model, which can determine risk factors which have had a significant effect on survival of cured and uncured patients (24). Since a proportion of individuals will survive from the event of interest, a cure rate model can be an appropriate and useful method to estimate cure fraction and model the rate of cured people directly (21). Cure models are categorized in two different major groups: The mixture and non-mixture cure fraction models. Non-mixture cure model or bounded cumulative hazard model are utilized to model both group, cure fraction, and survival parts of the model by specific approaches (25). The primary advantage of the non-mixture model is due to the fact that the proportional hazard assumption is held in this model. Moreover, according to recent studies, it has a biological interpretation. Indeed, in cancer-based studies, the survival time is defined as the result of a latent process, which is generating cancer tumors. Therefore, in the current study, the non-mixture cure rate model is used rather than the mixture cure rate model (26, 27).
The population cure was provided via the Kaplan-Meier curve for each category of categorical variables. Non-mixture cure models, which assumed various distribution such as log-normal, log-logistic, exponential, Rayleigh, and Weibull non-mixture cure rate model with logit link, were used to estimate cure fraction of patients and survival of patients with CRC. Akaike information criteria was utilized to compare the performance of parametric non-mixture cure rate. Since non-mixture cure rate models and Cox proportional hazards model characteristics are the same in multiple aspects including distribution form and proportional hazards assumptions, the stepwise selection was implemented on covariates to investigate if the best subset has the best fit on a Cox proportional hazard model and also removed multicollinearity in the model. According to this method, the subset was determined based on the variables of having P values less than 0.1 and more than 0.2 in our study. Variance inflation factor (VIF) was considered as a measure to check the presence of multicollinearity between the covariates. It is the measure of how much the variance of the estimated coefficient of the regression model is inflated by the existence of correlation among the predictor variables in the model. The best subset, which was obtained to fit Cox proportional hazards model, was chosen for the parametric non-mixture cure rate fitting.
The parametric non-mixture model estimates and Kaplan-Meier curve were used to drive (1) the observed proportion cured fraction of patients with CRC, (2) the median and mean survival rate of CRC for uncured patients, (3) the estimation of characteristics effect on cure fraction of patients with CRC, which was obtained, using maximum likelihood estimation (MLE) method on the individual patient records, and (4) the estimation of cure fraction of patients with CRC, using all covariates included in the non-mixture cure model.
The results were presented as the point estimate of characteristics’ impact of cure fraction from Weibull non-mixture cure model. The odds ratio was obtained by exponentiating the coefficients of variables. For categorical variables, the odds ratio demonstrates the odds of cure patients in each category compared to the baseline category. However, the odds ratio in the numerical variables shows changes in odds of cure patients by increasing one unit in the numerical variable. Meanwhile, the 95% confidence interval (CI) for each characteristic effect on the odds of cure patients was provided. The data were analyzed with the R software’s packages such as survival and bblme with 0.05 level of significance.
4. Results
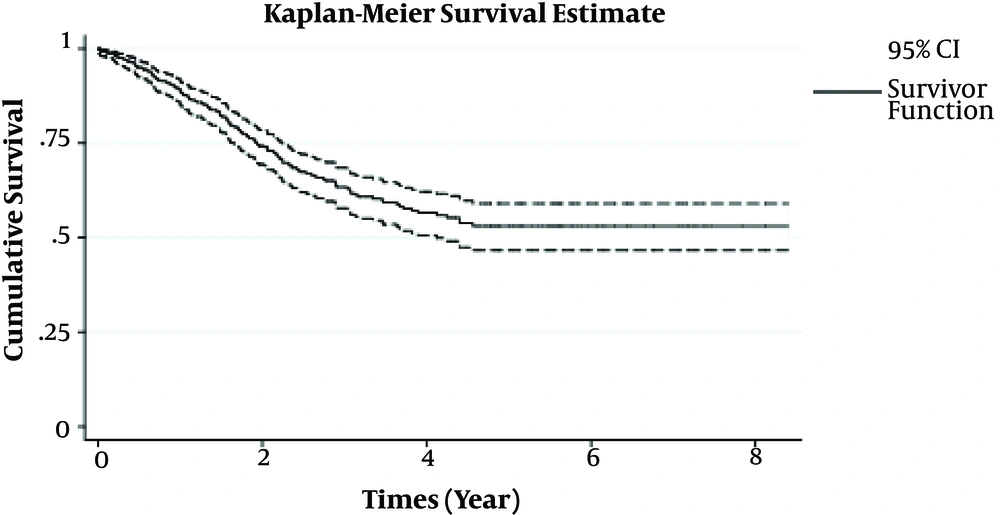
The following results were found in 512 patients with CRC. The mean (± standard deviation) age at the diagnosis of patients with CRC was 56.87 ± 14.27 years. For non-cured CRC patients, the survival time ranged from 0.01 to 4.56 years. The mean and median survival time in the non-cured group was 2.95 (95% CI: 2.76 - 3.13) and 3.08 (95% CI: 2.51 - 3.64) years, respectively. The 1, 3, and 5-year patients with CRC survival probabilities in non-cured group were 0.89 (95% CI: 0.86 - 0.92), 0.63 (95% CI: 0.58 - 0.69), and 0.53 (95% CI: 0.47 - 0.59), respectively. In the current study, 143 (28%) cases experienced death due to CRC and 369 (72%) were alive, censored, or dropped out by the end of the study. The patients’ characteristics and Log-rank test results are shown in Tables 1 and 2.
| Numerical Variables | Mean ± SD | Median (Q1 - Q3)a |
|---|---|---|
| Lymph node ratio | 0.14 ± 0.23 | 0.00 (0.00 - 0.205) |
| Age at diagnosis (y) | 56.87 ± 14.27 | 57.50 (47.00 - 66.00) |
| Size of tumor (cm) | 3.59 ± 2.50 | 3.50 (1.85 - 5.00) |
a Q1, first quantile; Q3, third quantile.
| Categorical Variables | Number of Patients | Number of Death | Log-Rank Test, P Valuesb |
|---|---|---|---|
| Site of tumor | 0.085 | ||
| Left colon | 31 (6.10) | 8 (5.60) | |
| Rectum | 199 (38.90) | 71 (49.70) | |
| Right colon | 86 (16.80) | 22 (15.40) | |
| Sigmoid | 196 (38.30) | 42 (29.40) | |
| Gender | 0.506 | ||
| Female | 223 (43.60) | 60 (42.00) | |
| Male | 289 (56.40) | 83 (58.00) | |
| Perineural invasion | 0.003 | ||
| No | 429 (83.80) | 109 (76.20) | |
| Yes | 83 (16.20) | 34 (23.80) | |
| AJCC cancer staging | 0.001 | ||
| I | 164 (32.00) | 33 (23.00) | |
| II | 159 (31.10) | 39 (27.27) | |
| III | 155 (30.30) | 54 (37.76) | |
| IV | 34 (6.60) | 17 (11.89) |
a Values are expresse as No. (%).
b Statistically significant: P value < 0.05.
After eliminating variables with VIF and implementing forward stepwise selection, site of the tumor, gender, perineural invasion, AJCC staging, lymph node ratio, age at diagnosis, and size of the tumor were selected as the best subset, which fit on Cox proportional hazard model. Figure 1 demonstrated that a stable plateau at the end of the study; thus, it is proved that there is population cure faction between patients with CRC. Therefore, using a Cox proportional hazard model was not appropriate. As shown in Figure 1, the Kaplan-Meier curve is stabled at the probability of almost 0.53, which implies that the overall population cure rate is 53% (standard error = 0.32, 95% confidence interval (CI: 0.47 - 0.60).
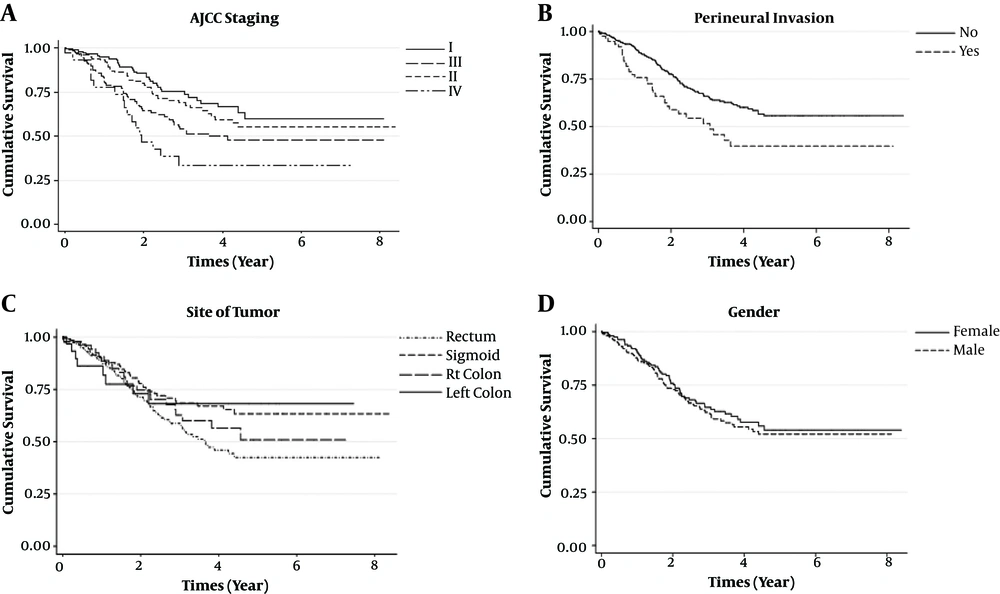
Also, according to the obtained results of Kaplan-Meier curves of the best subset in Figure 2, the cure fractions in females (0.54 with 95% CI: 0.45 - 0.65) and males (0.52 with 95% CI: 0.44 - 0.61) showed that females had a little more probability to be cured of CRC in comparison to males.
Patients with CRC with staging I (0.60 and 95% CI: 0.49 - 0.74) had the higher probability to cure CRC. Also, the probability of cure in patients with CRC with staging II (0.56 and 95% CI: 0.45 - 0.68) is so close to patients with CRC with staging I. Based on the findings of this study, the probability of cure in patients with CRC with staging III (0.48 and CI: 0.39 - 0.60), and staging IV (0.38 and 95% CI: 0.19 - 0.59) were lower than 50%. The curve of perineural invasion group indicated that the probability of being cure of CRC in the patients, who had perineural invasion (0.39 and 95% CI: 0.28 - 0.57), is lower than patients with CRC without perineural invasion (0.56 with 95% CI: 0.49 - 0.63). Patients with CRC, who had a tumor in their left colon, were less likely to die from CRC (0.68 with 95% CI: 0.52 - 0.90) in comparison to patients who had the tumor in their rectum (0.42 with 95% CI: 0.34 - 0.54).
According to the AICs from parametric non-mixture cure rate models, the Weibull non-mixture cure model outperformed other approaches (Weibull: AIC = 852.79, exponential: AIC = 863.50, log-logistic: AIC = 853.50, log-normal: AIC = 942.74, Rayleigh = 892.68). The obtained cure fraction from the Weibull non-mixture cure rate model was estimated to be 57.5%, which was reasonable compared to the overall population cure rate (53%).
All selected variables were entered into the model simultaneously. The results of the Weibull non-mixture cure rate model with logit link function are demonstrated in Table 3. Our analysis showed that AJCC staging, perineural invasion, and lymph node ratio had significant effects on cure fraction and survival of patients with CRC. According to the estimations, the odds of being cure from CRC in patients who were in staging IV was 85.00% lower than the patients in staging I (OR = 3.0.15, 95% CI: 0.043 - 0.498, P = 0.002). Also, the probability of being cure in patients with CRC with staging III were 59% lower than probability of cure in those with staging I. Odds of being cured of death in patients without perineural invasion was higher than patient in another group (OR = 0.41, 95% CI: 0.48 - 0.78, P = 0.007). The obtained result from Table 3 showed that for a one-unit increase in the lymph node ratio, the expected cure fraction of patients with CRC is reduced by 36% (OR = 0.64, 95% CI: 0.51 - 0.82, P = 0.002).
| Variable | Estimate | SE | OR | P Valuea | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Site of tumor | ||||||
| Left colon | ||||||
| Rectum | -1.018 | 0.577 | 0.361 | 0.077 | 0.117 | 0.622 |
| Right colon | -0.009 | 0.620 | 0.991 | 0.988 | 0.294 | 3.341 |
| Sigmoid | 0.282 | 0.563 | 1.326 | 0.616 | 0.440 | 3.999 |
| Gender | ||||||
| Female | ||||||
| Male | 0.061 | 0.272 | 1.063 | 0.824 | 0.624 | 1.811 |
| Perineural invasion | ||||||
| No | ||||||
| Yes | -0.892 | 0.333 | 0.410 | 0.007 | 0.213 | 0.915 |
| AJCC cancer staging | ||||||
| I | ||||||
| II | -0.561 | 0.359 | 0.571 | 0.118 | 0.282 | 1.153 |
| III | -0.881 | 0.397 | 0.414 | 0.026 | 0.190 | 0.902 |
| IV | -1.925 | 0.626 | 0.146 | 0.002 | 0.043 | 0.498 |
| Lymph node ratio | -0.440 | 0.122 | 0.644 | 0.002 | 0.507 | 0.818 |
| Age at diagnosis (y) | -0.006 | 0.008 | 0.994 | 0.452 | 0.979 | 1.009 |
| Size of tumor (cm) | -0.027 | 0.058 | 0.973 | 0.644 | 0.869 | 1.031 |
Abbreviations: CI, confidence interval; OR, odds ratio; SE, standard error.
a Significance at 5% level.
5. Discussion
In the current study, factors such as the site of the tumor, gender, perineural invasion, AJCC staging, lymph node ratio, age at diagnosis, and size of the tumor were determined as risk factors for colorectal cancer. In our study, approximately half of patients with CRC lived after a relatively long survival time.
The results in our study indicated that females have slightly higher odds of being cured of CRC than males, but gender had not any significant effect on survival and cure fraction of patients with CRC. On the one hand, previous studies have shown that females with CRC have better survival rates than men. Alternatively, these studies showed that CRC related factors were usually different in males and females (28). McArdle et al. found that patients with colonic tumors, those who underwent elective surgery and those who underwent apparently curative resection the overall survival at 5 years in women is higher than men. They also revealed that elderly females were more likely to have right-sided tumors and to be diagnosed emergently compared to males. Furthermore, their study demonstrated that females were less prone to have metastatic disease at the time of diagnosis (28). In another study, Amri et al. investigated challenges in ethnic and gender disparities in patients’ care. They demonstrated that females experienced more advanced stage, higher-grade disease, and lower rates of radical resection compared to males (29). Both of these studies have shown discrepancies in the various factors between males and females. Thus, gender could not be considered as an independent factor on the survival of patients with CRC. In the current study, there was no associations between gender and the stage of colorectal cancer. Gender did not have an independent effect on changing the cure fraction of patients. Therefore, to achieve more reliable results, some other factors including earlier detection should have been assessed in our study.
The stage is the most important variable in predicting the survival of patients with CRC. In the current study, the AJCC 5th edition cancer staging system was used as a factor to assess survival of patients with CRC. According to tumor-node-metastasis (TNM) classification on colon cancer, the AJCC 5th edition cancer staging system had only 4 categories (30). Based on this kind of staging category, the observed survival of patients with CRC with stage I and II was higher than 50%. Also, CRC patients’ survival rate with stage III and IV was lower than 50%. However, in our study, the survival rate of patients with CRC on stage IV was not very low. In similar studies, the survival rate of patients with CRC on stage IV was much lower than what we have achieved. For instance, in the study conducted by Oh et al. (31), on 365 eligible patients, the survival rate was reported 91% for stage I, 82% for stage II, 51% for stage III, and 4% for stage IV. Furthermore, in the current study, the effect size of AJCC staging on survival and cure fraction of patients with CRC was significant. Several studies including Li et al. and Chu et al. were in accordance with our study (32, 33).
The present study showed that the percentage of detecting cancer in the right-colon was more likely than the left-colon. Recently, similar results have been obtained in both western and eastern countries (34). In the study by Ishihara et al. (35), stage IV colon cancer patients followed patients from 1997 to 2007, it was found that stage IV right-sided colon cancer was more aggressive than left-sided colon cancer. In their cohort study, tumor location in the right-sided colon was associated with significantly worse cancer-specific survival (35). Although our study showed the non-significant impact of tumor location of cure fraction, previous studies have reported conflicting results about the right-sided and left-sided impact on patients’ survival (36, 37). Another study that was performed by Ishihara et al. at stage I-III colon cancer showed that right-sided colon had better survival rates than left-sided colon among CRC patients with mucinous adenocarcinoma and differentiated adenocarcinoma (38).
The current study has shown that the effect of Perineural invasion (PNI) on cure fraction of patients with CRC was statistically significant. Our findings validated the results of Liebig et al. study, which identified 269 consecutive patients who had CRC resected. Their analysis revealed that patients with CRC with positive PNI tumors were approximately twice as likely to experienced death due to CRC than their PNI-negative counterparts (39). Multiple studies revealed that in univariate and multivariate survival analysis, PNI-positive patients had lower overall survival than PNI-negative CRC patients. In addition, PNI was identified as an independent poor prognostic factor for assessing cancer specific overall survival (39, 40).
In the current study, we decided to investigate the effect of the lymph node ratio on the survival of CRC patients instead of the involved lymph node. Many studies have shown that the lymph node ratio is more precise than involved lymph node to predict the survival rate and it could be proposed as a candidate for using absolute number of affected lymph nodes in patients with CRC (41-45). The lymph node ratio was considered as a numerical variable in our study, which had a highly significant effect on cure fraction and survival of patients with CRC. These results coincided with findings in a study that was performed by Rausei et al. on patients who underwent CRC resection. Their findings demonstrated lymph node ratio as a simple and reliable tool to assess patients survival (46).
Recent studies have shown that tumor size is a prognostic factor for patients with CRC. In most studies, tumor size was used as a categorical variable with the optimal cut-off, which was determined by receiver operator characteristics (47, 48). The impact of the numerical form of tumor size in patients with CRC was investigated in the current study. Our findings indicated that this prognostic factor did not have any significant effect on survival and cure fraction of patients. This might be due to the use of tumor sizes in numerical form and the lack of appropriate cut-off values.
Although various studies have shown that increasing age is strongly associated with decreasing the CRC patients’ survival (49, 50). This prognostic factor had no significant effect on patients’ survival in the current study; this might be due to spreading of cancer in higher stages.
Due to significant improvement in the therapy of various types of cancer, the proportion of patients who are not susceptible to experience the event under study (the patients who are being cured) has increased (22). Since a proportion cancer cases may have long-term survival, cure rate model can be a proper method to identify and determine the potential risk factors that affect patients’ survival (21). In the present study, cure models were used to estimate the effect size of potential risk factors that affect cancer’ cure fraction.
The current study has a few limitations, which need to be considered when interpreting the results. We could not analyze some vital information, which was available in the medical records simultaneously. The simultaneous analysis of these factors led to statistical problems like the presence of multicollinearity between covariates.
While colorectal cancer is recognized as a fatal malignancy with a low survival rate in advanced stages, a substantial improvement on therapies for patients with CRC has been introduced in the recent years. Furthermore, appropriate survival analysis like the Weibull non-mixture cure rate model can help the clinicians and researchers identify potential risk factors, which affect the survival and cure fraction of patients who are not susceptible to death from CRC.
5.1. Conclusions
In this study, Perineural invasion, AJCC staging, and Lymph node ratio are determined as risk factors that affect both survival and cure fraction of patients with CRC. Perineural invasion should be assessed as an important factor in modeling survival analysis of patients with CRC. Given the shorter survival in patients who were in advanced metastasis stages of CRC, priority access to treatment and proper therapies is recommended. According to our finding, lymph node ratio was a proper tool to evaluate CRC patients’ survival.