1. Background
Curcumin, a yellow-colored hydrophobic polyphenol derived from the rhizome turmeric, has been used in medicine for centuries in Asia. Curcumin (Figure 1) (diferuloylmethane) significantly inhibited the growth of human breast cancer cells by inducing apoptosis in a dose- and time-dependent manner (1). This compound also suppresses Proliferation and Migration of breast cancer cells (2). As a result, curcumin can be used as a potential new treatment option for several cancers.
Fatty acid synthase is a multifunctional protein encoded by the FASN gene that catalyzes de novo synthesis of long-chain fatty acids. Lipid metabolism is mostly mutated in human carcinoma (3, 4). While most normal adult tissues use dietary lipids present by the blood stream, numerous cancers show enhanced amount of de novo fatty acid (FA) biosynthesis (5). The increased synthesis of fatty acids in tumor cells is essential for integration into membrane phospholipids and lipid signaling in constantly dividing cells.
FASN overexpression reasons resistance to multiple anticancer drugs via preventing treatment- induced ceramide production, caspase 8 activation, and apoptosis (6). Also, overexpression of FASN is intimately associated to progression of breast carcinoma and might be regarded as an independent prophesier of poor prognosis for breast carcinoma (7).
FASN expression is higher in HER2-positive cells, such as SKBR3 and MCF-7/HER2 cells, than in MCF-7 cells, which express lower HER2 levels (8, 9).
FASN inhibition that impedes the lypogenic pathway and obstructs fatty acid synthesis causes apoptosis in cancer cells that overexpress FAS, without affecting non‐malignant cells (10, 11).
Thus, targeting FASN has come to the consideration of many in the drug industries due to the discovery that the inhibition of de novo synthesis of fatty acids can be used to treat cancer patients. Since previous studies have shown apoptotic effects of curcumin via inhibition of FAS in only MDA-MB-231 human breast cancer cells and human hepatocellular carcinoma cell line (HepG2), we decided to study the effect of curcumin on FAS expression and activity in SK-BR-3 for the first time (12, 13).
2. Objectives
In this study, we decided to determine effect of Curcumin on fatty acid synthase expression and enzyme activity in breast cancer cell line SKBR3.
3. Methods
3.1. Chemicals and Materials
The analytical standard curcumin with 98.0% purity by HPLC was obtained from Sigma-Aldrich (St. Louis, MO, USA) and used in this study. Human breast cancer SKBR3 cells were obtained from pastor institute (Tehran, Iran).
Cell culture medium, Dulbecco’s modified eagle medium (DMEM), phosphate buffered saline (PBS) and fetal bovine serum (FBS) were obtained from Gibco laboratories (Grand Island, NY). MTT reagent was purchased from Sigma (St. Louis, MO, USA). RNA extraction kit was purchased from GeneALL Biotechnology Co. LTD (Seoul, Republic of Korea). Annexin V-FITC Apoptosis Detection Kit was purchased from eBioscience (San Diego, USA). RevertAid First Strand cDNA Synthesis kit was purchased from thermo scientific (Hudson, NH, USA).SYBR Green Master Mix was purchased from Takara (Japan). Ac-CoA, Mal-CoA, NADPH, and DMSO (Dimethyl Sulfoxide) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Cell Culture
Human breast cancer SKBR3 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) added with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C in a moistened atmosphere containing 5% CO2.
3.3. Cell Viability Assay
SKBR3 cells were plated in 96-well plates in triplicate with density of 5,000 cells per well. The medium was removed after 24 hours, and the cells were treated with curcumin (0, 2.5, 10, 15, 20, 25, 30 μM) for 24, 48, and 72 hours. MTT assays were used to evaluate cell survival. After the desired time, 10 mL of MTT (5.0 mg/mL) was added to each well and then the plate was incubated for 3 hours. The supernatants were aspirated carefully, and 100 mL of Dimethyl sulfoxide was added to each well to dissolve the crystals formazan made by the metabolism of living cells. The absorbance was measured by ELISA reader at 570 nm.
3.4. Apoptptic Analysis Using Flow Cytometric Assay
SK-BR-3 cells (200,000 cells/well) were seeded in triplicate in 6-well plate. After 24 hours, cells were treated with appropriate concentrations of curcumin (0, 5, 10, 15, 20, 25 μM) for 48 hours. Then the cells were collected and washed twice with phosphate buffered solution (PBS). To each microtube, 5 µM annexinV was added and placed in the dark after 5 minutes. 5 µM propidium iodide was added and they were again placed in the dark after 20 minutes. Incubation cells were analyzed by flow cytometry (Annexin V-FITC Apoptosis Detection Kit).
3.5. RNA Extraction and Real Time PCR
To extract total RNA, SK-BR-3 cells (600,000 cells/well) were seeded in 6-well plate. After 24 hours, the cells were treated with curcumin (0, 5, 10, 15, 20, μM) for 48 hours. Afterwards, RNA extraction was performed by kit (GeneALL). Total RNA was used to manufacture cDNA By RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). The expression of fatty acid synthase and ABL as a reference gene was examined by real-time PCR using the following primers: FAS forward, 5’-TGG TAG TGA GTG GGA AGG TGT AC-3’ and reverse, 5’-CAG ACG CAG CTC CTT GTA AAC TT-3’ and ABL forward, 5’-CTT CTT GGT GCG TGA GAG TGA G-3’ and reverse, 5’-GAC GTA GAG CTT GCC ATC AGA AG-3’.
3.6. FAS Activity Assay
After 48 hours of exposure to curcumin, cells were collected by treatment with trypsin-EDTA solution, pelleted by centrifugation and were washed twice with cold phosphate buffered solution (PBS). Cells were centrifuged at 13,000 rpm for 30 minutes at 4°C providing particle-free supernatants. Enzyme activity was measured based on changes in absorbance of NADPH at 340 nm. 50 μL Particle-free supernatant, 25 mM potassium Phosphate buffer, 0.25 mM EDTA, 0.25 mM dithiothreitol (DTT), 30 μM acetyl-CoA, and 350 μM NADPH (pH 7.0) in a total volume of 500 μL were Observed at 340 nm for 100 s to measure NADPH oxidation. After the addition of 100 μM of malonyl-CoA, the reaction was tested for an additional 1 minutes to designate FAS-dependent oxidation of NADPH (14). The change in concentration of NADPH during oxidation was measured using the following formula: ∆C = ∆A/E
Where ∆C is change in the concentration of NADPH, ∆A is the change in absorbance, and E is extinction coefficient of NADPH (E 340 nm = 6.22 mM-1cm-1). FAS activity was reported as nmol NADPH oxidized/min/mg protein (6).
3.7. Statistical Analysis
Data analysis was implemented using SPSS v.18.One-way ANOVA (with Dunnett’s multiple range test for post hoc comparison) was implemented to compare groups at diverse concentrations of curcumin. P < 0.001 was considered statistically significant.
4. Results
4.1. Effect of Curcumin on Cell Viability of SK-BR-3 Cells
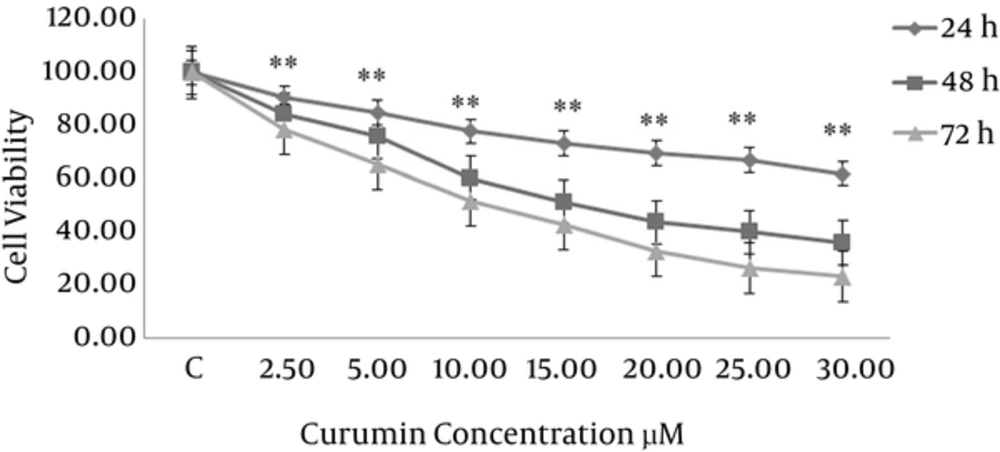
To assess the cytotoxicity of curcumin, breast cancer cells were treated with several concentrations of curcumin (0, 2.5, 5, 10, 15, 20, 25, 30 μM) for 24, 48 and 72 hours. Then cell viability was determined by a MTT assay. As shown in Figure 2 cytotoxic effect of curcumin on the growth of SK-BR-3 cells is dependent on time and concentration. The IC50 values of curcumin in SK-BR-3 cells were 15 μM in 48 hours and 10 μM in 72 hours.
SK-BR-3 cells were treated with different concentrations of curcumin (0, 2.5, 5, 10, 15, 20, 25, 30 μM) for 24, 48 and 72 hours. Then cell viability was determined by a MTT assay. The IC50 values of curcumin in SK-BR-3 cells were 15 μM in 48 hours and 10 μM in 72 hours. Each point is mean ± SD of three experiments; **P < 0.001 compared to control.
4.2. Curcumin Induced Apoptosis in SK-BR-3 Cells
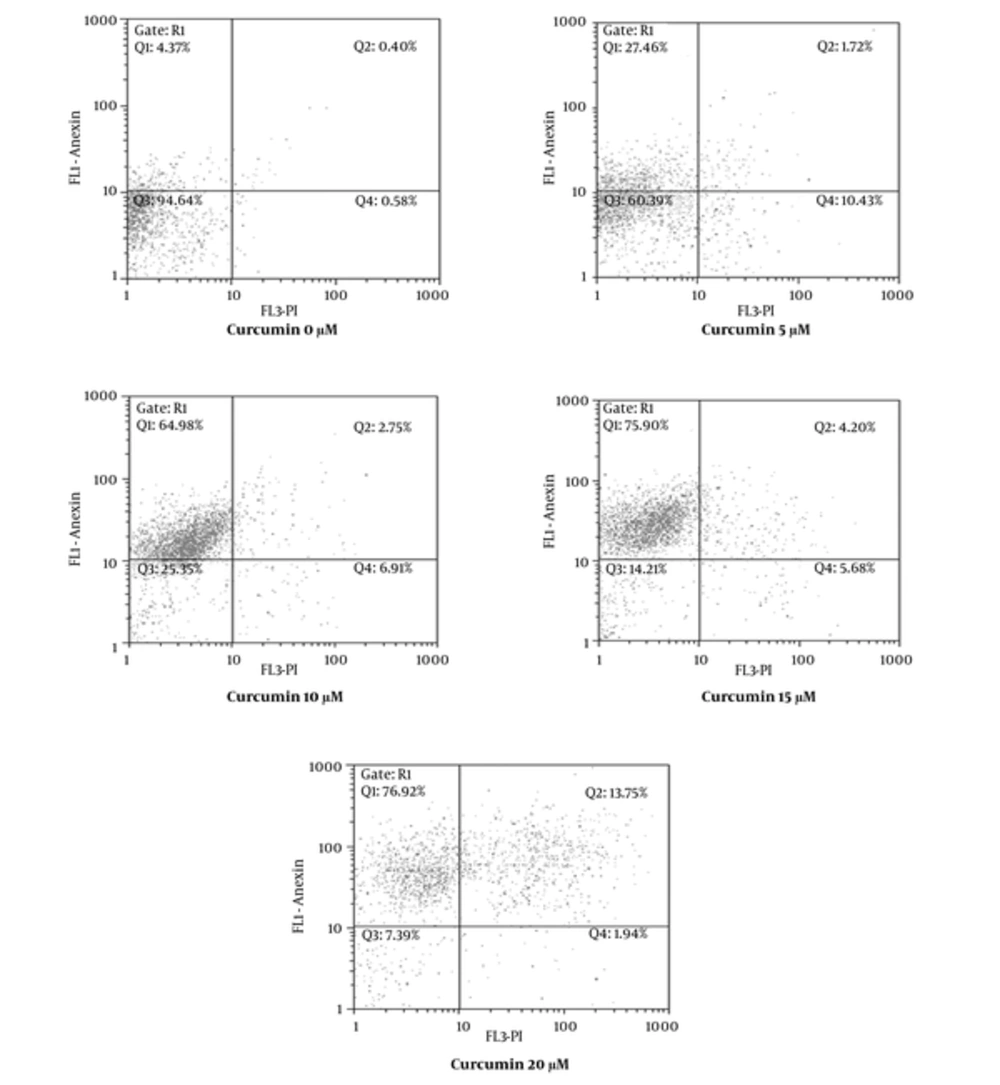
In order to verify whether the growth inhibitory effect of curcumin was connected with apoptosis, SK-BR-3 cells were treated with various concentrations of curcumin (0, 5, 10, 15, 20 μM) for 48 hours and afterwards they were examined by flow cytometry. As shown in Figure 3 cells were designated intact cells (Annexin V-, PI-), early apoptotic (Annexin V+, PI-), damaged (Annexin V–, PI+) and late apoptotic (Annexin V+, PI+). The early apoptotic rate in SK-BR-3 cells treated with curcumin was 4.37% at 0 μM, 27.46% at 5 μM, 64.98% at 10 μM, 75.90% at 15 μM and 76.92% at 20 μM. According to the results, curcumin induced apoptosis in SK-BR-3 cells in a dose-dependent manner.
4.3. Curcumin Suppressed FAS Expression and Inhibited Fatty Acid Synthase Activity in SK-BR-3 Cells
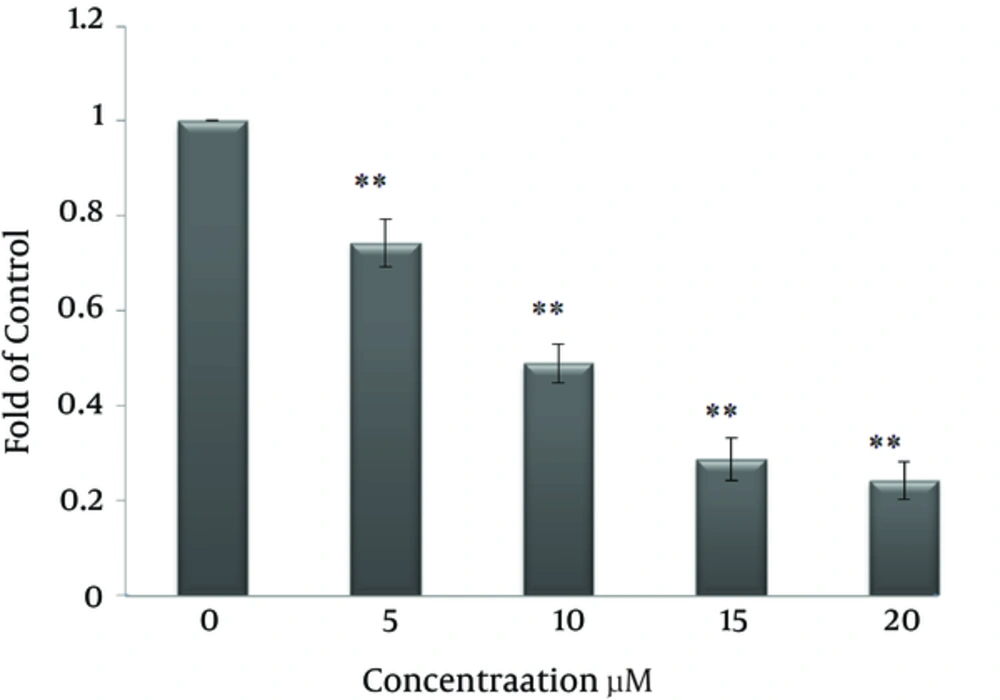
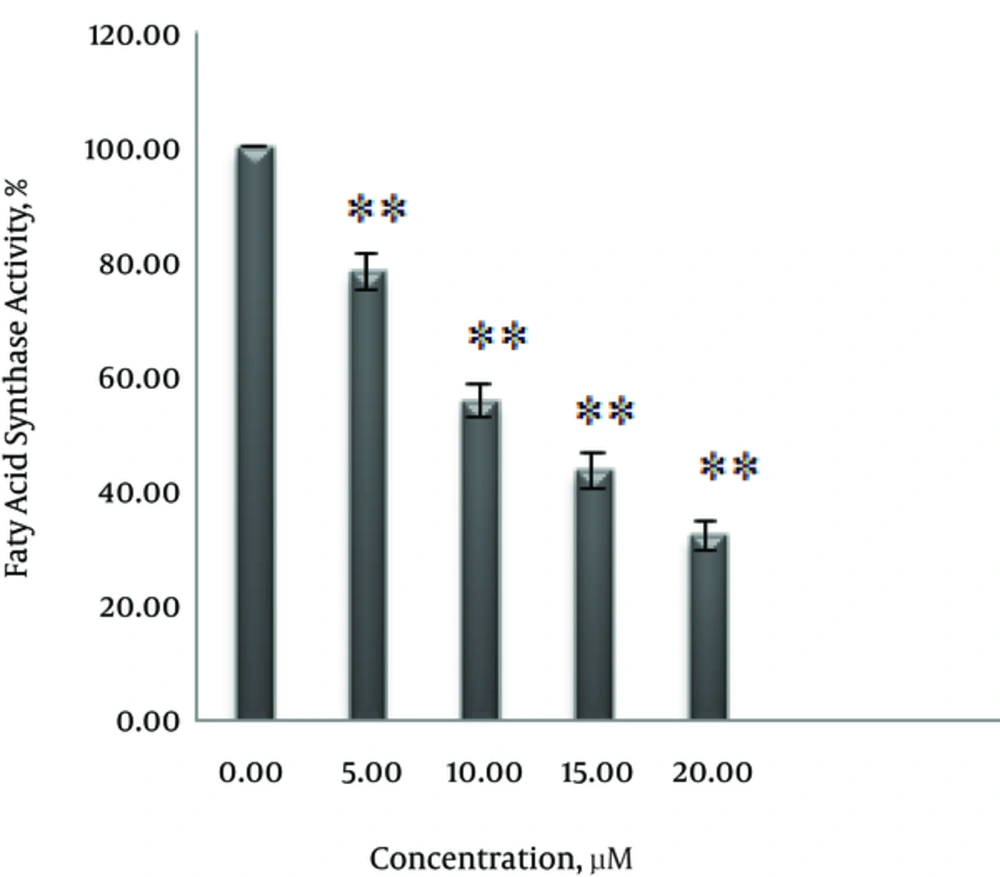
To explore the effect of curcumin on FAS expression and fatty acid activity, SK-BR-3 cells were treated with 0, 5, 10, 15 and 20 μM curcumin for 48 hours. As shown in Figure 4 curcumin decreased expression of FAS in a dose-dependent manner. Also, the inhibitory effect of curcumin on fatty acid synthase activity, according to the results in Figure 5 depends on concentration. Enzyme activity decreased by 21.7% at 5 μM, 41.4% at 10 μM, 56.4% at 15 μM and 67.7 % at 20 μM.
5. Discussion
Despite various treatments, breast carcinoma is the most common cancer and a leading cause of mortality among females worldwide (15, 16). Fatty acid synthase is a multifunction protein that catalyzes de novo synthesis of long-chain fatty acids. Fatty acids play an important role in critical functions including reproduction, energetic metabolism through β‐oxidation, signal transduction, DNA synthesis, intracellular trafficking, protein acylation, cell polarization, cell cycle progression, and cell migration (17-19). Fatty acid synthase expression in tumor cells and normal cells were different; therefore, fatty acid synthase enzyme was used as a therapeutic target in the improvement of anticancer drugs (20-23). FAS inhibition reduced the production of lipids in the cancer cells and induced apoptosis in overexpressed FAS cancer cells, without affecting non-malignant cells (10). Overexpression of FAS is common in a wide range of cancers, especially breast cancers (24, 25). Increased fatty acids synthesis in tumor cells is essential for integration of phospholipids into the membrane and lipid signaling in constant cell divisions (17, 18, 21, 26). Curcumin, a yellow-colored hydrophobic polyphenol derived from the rhizome turmeric, has anti-cancer effects in vitro and in vivo on several types of cancers (27).
As shown in Figure 2 in our study, the IC50 values of curcumin in SK-BR-3 cells were 15 μM in 48 hours and 10 μM in 72 hours which indicated that curcumin reduced cell proliferation of cancer cells in a dose- and time-dependent manner.
Our results are in agreement with many studies (1, 28-31) for instance in the study conducted by Yan et al. with IC50 of 20 μM was obtained at 48 hours of treatment by curcumin in SK-BR-3 cells (29). Furthermore, consistent with the research by Plalange et al. curcumin with concentrations less than 30 μM had no significant effect on viability of cancer cells in 24 hours (31).
However, our results were in contrast with the results reported by Thomas who showed that the IC50 of curcumin was 3.0 ± 0.5 μM in 24 hours in SK-BR-3 cells.
The inhibitory effect of curcumin on the fatty acid synthase enzyme were previously observed in certain cell lines (12, 13). However, in this study for the first time, the inhibitory effect of curcumin on the expression and activity of fatty acid synthase in SK-BR-3 cells were examined. Following treatment with curcumin, expression and activity of fatty acid synthase were reduced in a dose-dependent manner (Figures 4 and 5). 5 μM, 10 μM, 15 μM and 20 μM concentrations of curcumin reduced enzyme activity by 21%, 41%, 56.4% and 67.7%, respectively.
In this study, curcumin with a concentration of 5 μM reduced enzyme activity by 21% in SK-BR-3 cells. While in human breast cancer MDA-MB-231 cells, enzyme activity was reduced by 6.8% at 5 µM concentration of curcumin (12). As a result, curcumin in breast cancer cell line SK-BR-3 compared with MDA-MB-231 cells is more capable of inhibiting the activity of fatty acid synthase.
Several studies have shown that inhibition of FAS may induce cell apoptosis via reducing fatty acids (12, 14, 32). The results obtained by the Annexin V/PI dual staining in our study suggests that curcumin induced apoptosis dose-dependently (Figure 3). 20 µM of curcumin concentration caused apoptosis in SK-BR-3 cells after 48 hours by 76.92%, while in contrast with our results in the study conducted by Guang Yan et al. curcumin, with concentration of 20 µM for 48 hours, had no significant effect on apoptosis of SK-BR-3 cells (29).
These results support the significant role of FAS in SK-BR-3 cells and propose that FAS is the aim that curcumin act on.
5.1. Conclusions
In summary, curcumin could induce apoptosis in SK-BR-3 cells. Curcumin treatment down-regulated the expression of FASN and inhibited fatty acid synthase activity in SK-BR-3 cells.
Therefore, it is possible that inhibitory effect of curcumin on FAS may induce apoptosis in human breast cancer SK-BR-3 cells.