1. Background
Many studies have shown that CD8+ T lymphocytes can destroy cancer cells, so most approaches in cancer immunotherapy have focused on the induction of these cells against tumor antigens (1). Accordingly, researchers have tried to identify the peptides prompting cytotoxic T cell (CTL) responses. However, this approach has not been effective in clinical trials, probably due to lack of other effector T cells, such as T helper (Th) and memory cells (2). On the other hand, recent studies have shown that CD4+ T cells can maintain an effective immune response against tumors through interactions with antigen-presenting cells (APCs) and ongoing activation of CD8+ T cells (3). The pivotal role of CD4+ T cells in harnessing the tumor growth and inducing protection against tumors was proved in MHC II-deficient mice. Noguchi et al. demonstrated that transferring adaptive virus-specific CD4+ T cells, inducing FBL-3 (MuLV) tumor can control tumor growth in a CTL-independent path (4). The potency of CD8+ T cells is strengthened via the insertion of MHC Class II epitopes in peptide-based vaccines (2). Also, Th cells have a lethal function that helps the host defense system overcome tumor progression (5). Also, these cells prompt executive innate immune cells such as macrophages and NK cells through cytokine secretion (6). In the field of cancer immunology, a plethora of evidence supports the significance of CD4+ T cells in memory cell excitation. For example, Qiu Zhengrong et al. (2007) demonstrated that MHC class II-restricted Th cell responses are required to generate memory CTL induced by a Poly(I:C)-adjuvant MHC I-restricted peptide (7). CD4+ T cells control the growth and presence of antigen-specific CD8+Tcells in the body. This action occurs through the secretion of essential cytokines such as interleukin-2 (IL-2) in the presence of CD8+ T cells (4). Many studies have shown that immunization with specific CD8+ T cell peptides alone can cause an antigen-specific cytotoxic response with a short lifespan and no memory cells (8). Therefore, to create a complete and prolonged anti-tumor response and eventually, the complete destruction of the tumor, the participation of both CD8+ and CD4+ anti-tumor T cells is required (9). In this regard, many studies have used databases to identify peptide epitopes for stimulation and induction of CD4+ T cell responses. These peptide epitopesinclude MAGE-3, MART-1, NYESO, and tyrosinase (10-14). Schroers et al. (2003) demonstrated that human telomerase reverse transcriptase (hTERT) 766 can be presented by class II MHC alleles including DR4, DR11, and DR15 (15). Thus, in this study the induction of a CD4+ T cell response by a peptide obtained from hTERT enzyme according to HLA-DRB1*11:03/04 (HLA dominant in Iran) was studied. According to Schmitt et al. (1996) viral peptides can stimulate immune responses against tumor cells. Therefore, in this study, we used viral peptides in combination with a tumoral peptide to strengthen the anti-tumor immune response.
2. Methods
2.1. Epitope Prediction and Peptide Synthesis
Various viral and tumoral peptides were examined according to a common HLA in Iran (HLA-DRB1*11:03/04), literature review, and databases, including EPIMHC (http://imed.med.ucm.es/epimhc/), SYFPEITHI (http://www.syfpeithi.de/), IEDB (http://www.iedb.org/home-v2.php), and the HIV database (http://www.hiv.lanl.gov/content/index). The viral peptide selection criteria were based on the HIV Database and HLA-DRB1*11:03/04, ability to be processed and presented by APCs, and immunogenic potential Peptides containing 16 amino acids of the HIV vpr protein were selected (16).
Many articles were examined to identify a tumor peptide that is restricted to HLA-DRB1*11:03/04, however, because of the commonness of this HLA in Iran, no reports of a peptide restricted to this HLA were found. Therefore, peptidesfrom HLA-DRB1*11, which are similar to those in HLA-DRB1*11:03/04, were selected and evaluated. Consequently, a 15 amino acid tumor peptide from hTERT was selected (15). Lyophilized peptides were provided by Selleckchem, US.
2.2. Blood Samples and HLA Typing
Whole blood samples were taken from 30 patients diagnosed with non-small cell lung cancer (provided from Shariati hospital, Tehran, Iran) and 70 healthy subjects (from the students of Tehran University). The patients and healthy subjects have been matched by sex and age. HLA typing was performed by PCR-sequence-specific primer DNA-based procedures and primers from previous studies were used for screening the HLA-DRB1*11:03/04. The forward primer was 5’GTTTCTTGGAGTACTCTACGTC3’ and the reverse primer was 5’CTGGCTGTTCCAGTACTCCT3’ (17). Ten ml of heparinized blood was collected in sterile conditions from HLA-positive subjects and PBMCs were isolated in a Ficoll gradient. Informed consent was acquired from all subjects according to the guidelines from the Tehran University research ethic committee. The ethical committee of immunology, asthma and allergy research institute, Tehran University of Medical Sciences approved the study’s protocol.
2.3. Cell Lines
The tumor cell line A549 (NCBI, Pasteur Institute of Iran) was used to examine the production of IFN-γ from CD4+ T cells, which were treated with peptides while co-cultured with the cancer cells. A549 cells originated from lung adenocarcinoma epithelial cells and are HLA-DRB1*11:03/04 positive. Rha et al. (2006) confirmed hTERT expression in this cell line (18).
2.4. In vitro Stimulation of PBMCs
Donor PBMCs were plated in U-bottomed 96-well plates at 200,000 cells/well in RPMI 1640 media at 37°C in 5% CO2. Peptides and controls were divided into four groups: negative control, tumoral, tumoral plus viral peptide, and positive control. To gain the most reliable determination, the MTT assays were performed in triplicate for each group. Tumor and/or virus peptides were added to well at 20 μg/mL for 5 days. 1×104 A549 cells, whose growth was stopped by 2 μg/mL mitomycin, were then added to each well and incubated for 72 hours. In the last 24 hours of incubation, 10 µg/mL PHA was added to the cells of one group as a positive control. Then, 20 μL of 5 mg/mL MTT stock were added to each well. After four hours of incubation at 37°C, 100 μL of MTT solvent was added to each well and the optical density (OD) were read on an ELISA plate reader at 570 nm and a reference wavelength of 630 nm. Before the MTT assay, 100 μL of cell supernatant was collected for the IFN-γ assay.
2.5. Evaluation of T-Cell Responses by IFN-γ ELISA Assay
IFN-γ from the TCD4+ T cells treated with peptide in the presence of cancer cells was measured using an ELISA kit (R and D, USA) according to the manufacturer’s instructions.
2.6. Statistical Analysis
Statistical analysis of all obtained data are reported as means ± SDs. Significant differences between the data were assessed by the Mann-Whitney U test in the ELISA and Student’s t-test in the MTT assays. The Pearson correlation test was used for correlation analysis. All statistical analyses and correlation graphs were performed and produced using SPSS 11.5 and GraphPad Prism software. P values < 0.05 were considered significant.
3. Results
3.1. Primary Identification of MHC Class II Restricted Epitopes
The tumor peptide selected from hTERT (766 - 780) contains the amino acid sequence LTDLQPYMRQFVAHL. The LQQLLFIHFRIGCRHS peptide from the HIV vpr protein (64 - 79) was chosen from the HIV database. The peptides were checked for undesired binding to MHC I. All scores were low in this regard.
3.2. PCR Test Results to Determine the HLA-DRB1*11:03/04
Twenty-one of 100 samples were HLA-DRB1*11:03/04 positive. Samples from six lung cancer patients (59.3 ± 10.93) and nine healthy individuals (28.7 ± 3.83) were used in the study.
3.3. Proliferation Assay
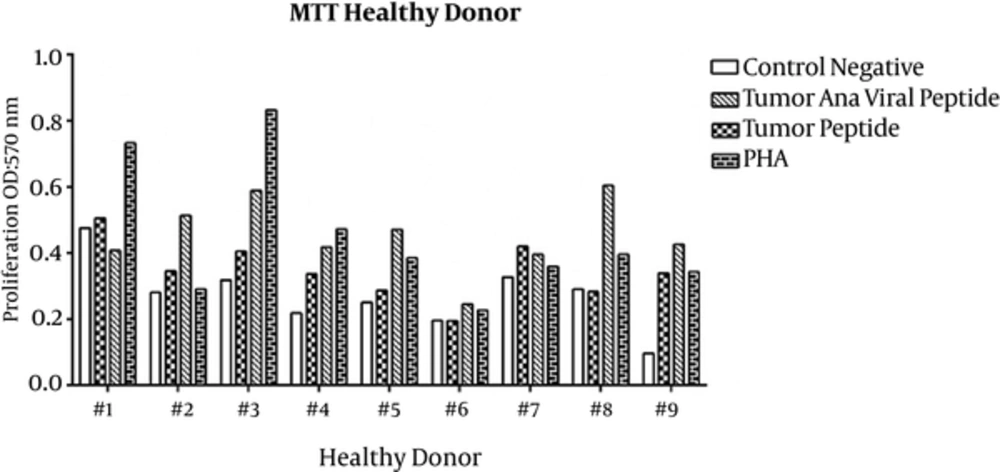
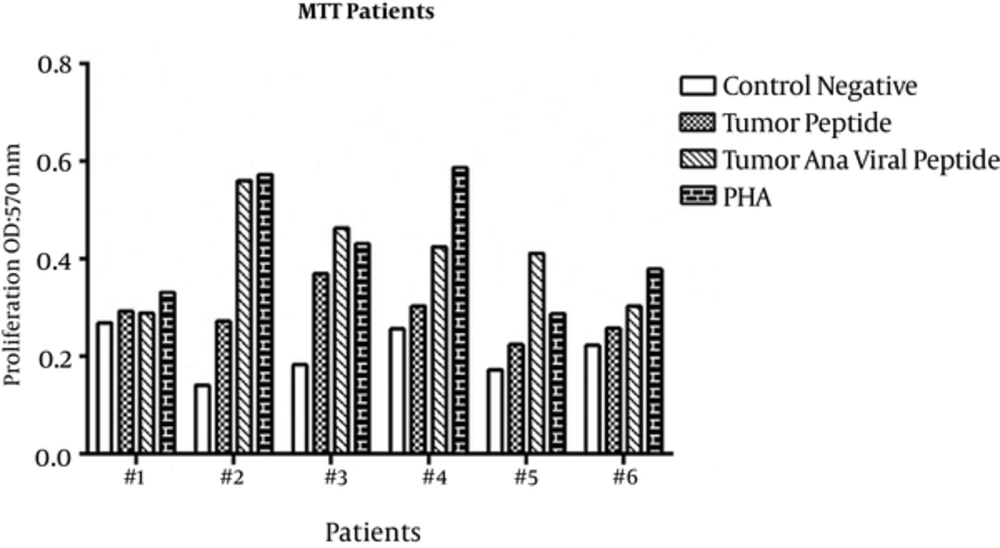
Proliferation of PBMCs treated with tumoral and viral peptides in the presence of A549 cells were evaluated by MTT assay. The OD results of the MTT assay are shown in Figures 1 and 2 for healthy donors and patients, respectively, and means ± SDs are presented in Table 1.
| Variables | Negative Control | Tumoral Peptide | Tumoral Plus Viral Peptide | Positive Control |
|---|---|---|---|---|
| MTT | ||||
| Healthy Ind. | 0.270 ± 0.097 | 0.349 ± 0.101 | 0.452 ± 0.133 | 0.448 ± 0.213 |
| Lung cancer patients | 0.207 ± 0.075 | 0.278 ± 0.100 | 0.407 ± 0.110 | 0.449 ± 0.151 |
| IFN-γ | ||||
| Healthy Ind. | 301.88 ± 234.43 | 468.55 ± 153.71 | 719.66 ± 258.16 | 620.77 ± 239.62 |
| Lung cancer patients | 461.33 ± 134.07 | 631.33 ± 196.51 | 818.00 ± 250.65 | 894.66 ± 252.69 |
Means ± SD of MTT and ELISA Analysis for all Samplesa
Proliferation of PBMCs stimulated with tumoral peptide alone was statistically significant than negative controls (P = 0.001). Also, proliferation of PBMCs stimulated with tumoral plus viral peptide was statistically significant (P = 0.000) than negative controls. Proliferation of PBMCs stimulated with tumoral plus viral peptide was significantly greater than PBMCs stimulated with tumoral peptide alone (P = 0.000).
3.4. The IFN-γ Secreted by PBMCs
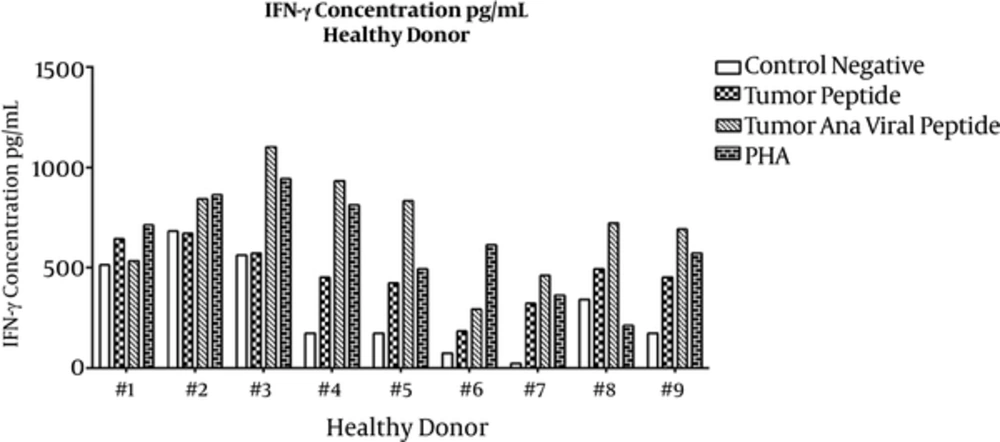
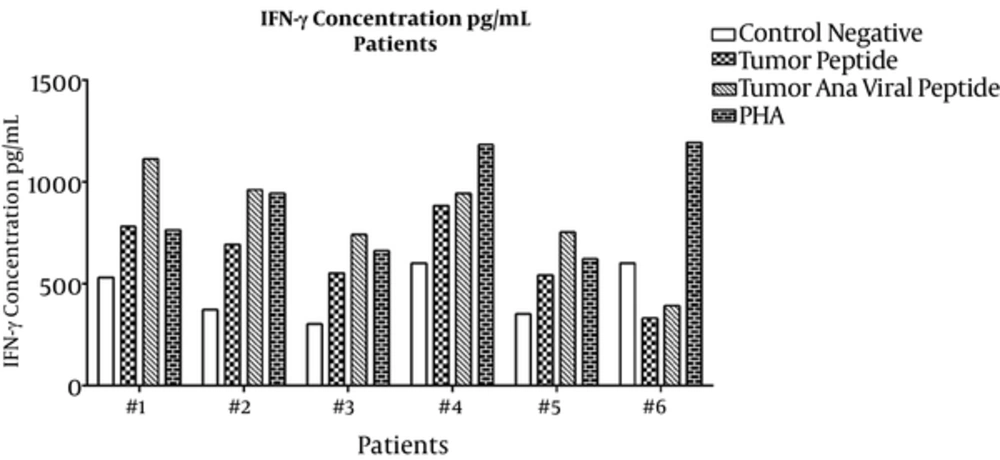
For the IFN-γ assay, culture supernatants were collected on the last day of incubation and sandwich ELISA was performed. Means ± SDs are presented in Table 1. ELISA results for individual healthy subjects and lung cancer patients are shown in Figures 3 and 4, respectively. IFN-γ secretion from PBMCs treated with tumoral plus viral peptides was greater than PBMCs treated with the tumoral peptide alone (P = 0.009).
The results showed a significant difference in the secretion pattern of IFN-γ in tumor peptide and tumoral plus viral peptide groups with the negative group (respectively P= 0.000 and P = 0.028).
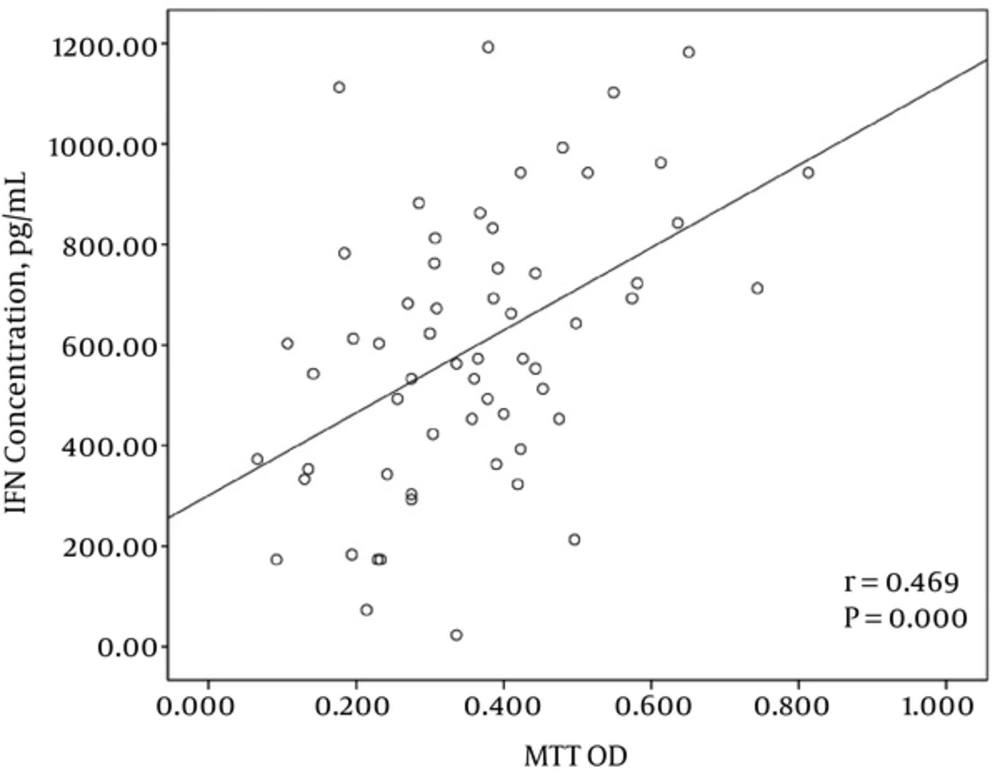
3.5. Correlation Between Proliferative Response and IFN-γ Secretion
The correlation analysis between the proliferative response of PBMCs and IFN-γ production after stimulation with tumor plus viral peptides showed a significant correlation between these two parameters (r = 0.469 and P = 0.000) (Figure 5).
4. Discussion
In this study, we demonstrated that tumor peptide derived from hTERT can induce CD4+ T cell proliferation and IFN-γ secretion in both healthy subjects and lung cancer patients in the presence of A549 cells. Our data agrees with the results of Schroers et al., in which the derived tumor peptide was carried out by HLA-DRB1*11 (15). They selected peptides based on the relevant HLA to induce CD4+T cells. HTERT peptide (position 766) were bound to HLA-DRB1*11 with the highest immunogenicity and induced CD4+T cell proliferation in transgenic mice (15). According to the results shown in table 1, the average proliferation in patient-derived PBMCs was slightly less than that of healthy individuals. This reduction might be due to the presence of previously-activated T regulatory cells. Tumor cells activate regulatory cells to evade the immune system. In patients with lung cancer, production of COX-2, PG-E2, NSCLC, and over-expression of FOXP3 transcription factor lead to an increase in T regulatory cells (19). This shows the importance of activated CD4+ T cells as effector cells rather than T regulatory cells.
Peptides restricted to MHC class II make only the CD4+ T cell response. CD4+ T cells induce memory responses in specific CTLs to prevent tumor recurrence (20). Ait-Tahar et al. (2010) showed that using the PASD-1 peptide in patients activated CD4+ T cell responses against the peptide resulting incomplete tumor regression. The main point of that study was that CD4+ T cell clones created in a previous step lysed tumor cells expressing the PASD-1 tumor antigen (5). Another vaccination strategy used transduced tumor cells with AIR-1-encoded class II MHC transactivator (CIITA). CIITA has been investigated in a study to gain ideal T cell responses against tumor cells. In this study, tumor cells were rejected at a high level in immunocompetent syngeneic mice that had been injected with CIITA-transfected tumor cells (21). The use of xenogeneic or non-tumor antigen like PADRE (derived from keyhole limpet hemocyanin) and the tetanus-derived toxoid peptide are common approaches to stimulate helper T cells to provide non-specific help and recall immune memory (22). Buschle et al. showed that viral peptides can stimulate immune responses against tumor cells (23). Findings achieved from PBMCs treated with both tumor and viral peptides indicate an increase in proliferation and IFN-γ secretion compared to the negative controls and stimulation by tumoral peptide alone. These results show that the viral peptides can stimulate CD4+ T cells as effector cells.
Strategies that use the immune system to treat cancers face problems with regard to individual differences in the immune responses and tumor types. Each patient’s immune system is uniquely variable in terms of genetics, nutrition, lifestyle, and other factors (24). Furthermore, because of tumor dissimilarities, certain cancer treatments may benefit only small patient subsets. Response heterogeneities are believed to be related to HLA genes, cytokines and chemokines and their receptors, costimulatory molecules and their ligands, and toll-like receptors (25). The major goal of this study was to induce tumor antigen-specific CD4+ Th cells. To achieve this goal, we utilized specific class II MHC-binding tumoral and viral antigen peptides as a personalized vaccine. Because different peptides are presented by distinct polymorphic class II molecules, patients are usually HLA typed so that the appropriate peptide can be utilized. The most commonly expressed class II MHC allele in Iranian populations is HLA-DRB1*11:03/04, thus most clinical trials have focused on this patient subset. In parallel, most trials have analyzed patient tumors for antigens of interest. HTERT antigen is one of the best choices for designing anti-tumor vaccines without the activation of the immune system against normal cells. HTERT is regularly over-expressed in more than 85% of tumors compared with normal cells (26). Therefore, suitability for such studies requires both expression of the defined antigen by the tumor, and the presence of the specific HLA allele in the host.
Therefore, to make a long-lasting and effective response and ultimately, to completely eradicate the tumor, the participation of both tumor-specific CD8+ and CD4+ T cells are needed. In subsequent studies, we suggest that peptides restricted to class I and II HLA, accompanied by viral peptides, be applied in in vitro and in vivo conditions. Our future studies will focus on this issue.