1. Background
Nowadays, different cancers including oral cancer are serious threats to human health (1, 2). Oral cancer is one of the most prevalent malignancies and presents increasing incidence and mortality trends in the world with a mortality rate of 14.33% (3). Oral squamous cell carcinoma (OSCC) constitutes 90% of all malignancies in the oral cavity. In spite of new therapeutic strategies, the risk of metastasis and mortality rate is still high (1, 3-7). Therefore, it is urgent to understand the molecular mechanisms of OSCC tumorigenesis and metastasis. Several molecular signaling and pathways contribute to the development and metastasis of OSCC (8).
Endocan, also called endothelial cell-specific molecule-1 (ESM-1), is a kind of secreted dermatan sulfate proteoglycan that is related to the tumorigenesis and tumor progression (9, 10). Endocan is a newly diagnosed endothelial cell activation marker (11). Previous studies suggested that altered endocan expression level on tumor vessels reflects the angiogenesis and invasion in cancers (11). In several cancers, the clinical significance of endocan expression is controversial. While some published works considered endocan as a marker for angiogenesis, others considered it as a prognostic factor (12-15). The overexpression of endocan in some cancers such as lung cancer, kidney cancer, and acute leukemia enhances its aggressive behavior (12, 16, 17), however; in gastric cancer and colon cancer, endocan functions as a tumor suppressor (18, 19). Endocan is a predictor of cancer recurrence in patients with pancreatic neuroendocrine tumors (20). According to a previously published article, the endocan expression has been demonstrated in cancer cells (21).
Tumor growth and metastasis are correlated to the function and the altered expression level of many proteins, as well as gene mutations (5, 8, 22-24). In addition, cancer cell invasion and metastasis are correlated to the level of cancer cell differentiation. Previous studies revealed that the lower the differentiation level, the more abilities of cancer cell migration, invasion, and metastasis. In addition, angiogenesis is critical for cancer progression and metastasis; hence, the number of vascular channels increases in cancer tissues to provide adequate nutrition for cancer cells (23, 25-27). Several molecules and signaling factors are involved in angiogenesis in cancers. Besides, in highly aggressive cancers, tumor cells form vascular-like channels lined by tumor cells instead of endothelial cells called vasculogenic mimicry (VM), which participates in tumor progression and metastasis (8, 23, 28). The head and neck area is rich in lymphatic vessels and lymph node metastasis is a common event in head and neck cancers (5, 22, 29, 30). So far, the precise function of endocan in human OSCC remains unclear, but it had been suggested that endocan regulates invasion and metastasis in murine OSCC (31). Investigations on the role of endocan in human oral cancer cells may result in the use of endocan as a therapeutic and prognostic tool. Accurate techniques are needed for early detection and immunohistochemistry (IHC) is one of these techniques (23).
2. Objectives
The present study aimed at demonstrating the expression level of endocan protein in human OSCC by using the IHC technique.
3. Methods
This study was evaluated and accepted by the Ethics Committee of Hamadan University of Medical Sciences (Institutional Review Board approval number Res. Proj.9504222160). A total of 54 samples (18 samples of each grade) were collected from the archive of Pathology Department of Be’sat Educational Hospital, Hamadan, Iran from 2000 to 2017. Hematoxylin and eosin (H&E) staining was performed to confirm the previous diagnosis.
3.1. Immunohistochemistry Staining
The specimens were processed for IHC analysis. The monoclonal anti-mouse antibody used in the IHC assay was ESM1 (1:170; Abcam; 56914). Briefly, the paraffin blocks were sliced into the 4 µm thick sections. Then, the sections were deparaffinized and dehydrated with graded alcohol. The antigen retrieval was done in citrate buffer (pH = 6). By using the Leica detection kit, endogenous peroxidase activity was blocked. After 3 washes in Tris-buffered saline (TBS), the samples were incubated with primary antibody for 1 hour. Negative control was prepared by omitting the primary antibody. After TBS washing, the slides were developed in the freshly prepared 3, 3’-diaminobenzidine solution (DAB) for 6 minutes, followed by counterstaining with hematoxylin, dehydration, and mounting. In addition, the slides were stained by the PAS method to evaluate VM formation. Microvessel density (MVD) was identified by the light microscopic examination of stained sections at the “hot spot”. The fields of the greatest neovascularization were selected by light microscope at low magnification (X100). The average vessel count of the 5 fields (× 400) was regarded as the MVD. The MVD was classified as either high (≥ 15) or low (< 15); 15 was considered as the median value of MVD in our study (8, 23).
3.2. Detection and Scoring
The endocan expression level was evaluated in the cytoplasm of tissue sample cells. The immunostaining was assessed via quantitative methods. Five photomicrographs of each sample were acquired. The abundance of positive cells was graded as follows: 1 (weak) for < 20% positive cells, 2 (moderate) for 20% to 50% positive cells, and 3 (strong) for > 50% positive cells (30).
3.3. Statistical Analysis
The statistical analysis was performed by the Statistical Package for Social Sciences software version 22.0 (Chicago, IL, USA). The data were analyzed, using chi-square test to determine whether there was a significant difference between the comparing groups regarding the endocan expression level.
4. Results
A total of 54 samples were used for the immunohistochemical study. The chi-square test showed statistically significant differences between the expression level of endocan and lesion grade in the research groups (P = 0.025), lesion grade and VM formation (P = 0.001), and tumor grade and MVD count (P = 0.000). According to the Spearman’s rank correlation analysis, tumor grade had a positive correlation with the endocan expression level (Pearson’s r = 0.363, P < 0.007), with VM formation (Pearson’s r = 0.512, P < 0.000), and MVD count (Pearson’s r = 0.488, P < 0.000). The results of the chi-square test are summarized in Table 1.
| Variables | Low Grade Tumor | Intermediate Grade Tumor | High Grade Tumor | P Value |
|---|---|---|---|---|
| Endocan expression level | 0.026* | |||
| Moderate | 13 (72.2) | 8 (44.4) | 5 (27.8) | |
| Strong | 5 (27.8) | 10 (55.6) | 13 (72.2) | |
| VM formation | 0.001* | |||
| Positive | 5 (27.8) | 12 (66.7) | 16 (88.9) | |
| Negative | 13 (72.2) | 6 (33.3) | 2 (11.1) | |
| MVD count | 0.000* | |||
| < 15 | 12 (66.7) | 3 (16.7) | 2 (11.1) | |
| ≥ 15 | 6 (33.3) | 15 (83.3) | 16 (88.9) |
aValues are expressed as No. (%).
5. Discussion
OSCC, the most common primary cancer of the oral cavity, is associated with a high incidence and mortality rate around the world. Early detection, diagnosis, and treatment play vital roles in the reduction of mortality rate (8, 32). Additionally, the development of new biomarkers and signaling pathways has a great impact on early cancer detection (33). Endocan is one of the most important molecules detected in several types of cancer and its altered expression level is correlated with the degree of tumor differentiation (20, 34). Cell differentiation is critical for normal growth and dedifferentiation is a driver of cancer development (35). The current study investigated the possible involvement of endocan in the differentiation and invasion of OSCC cells.
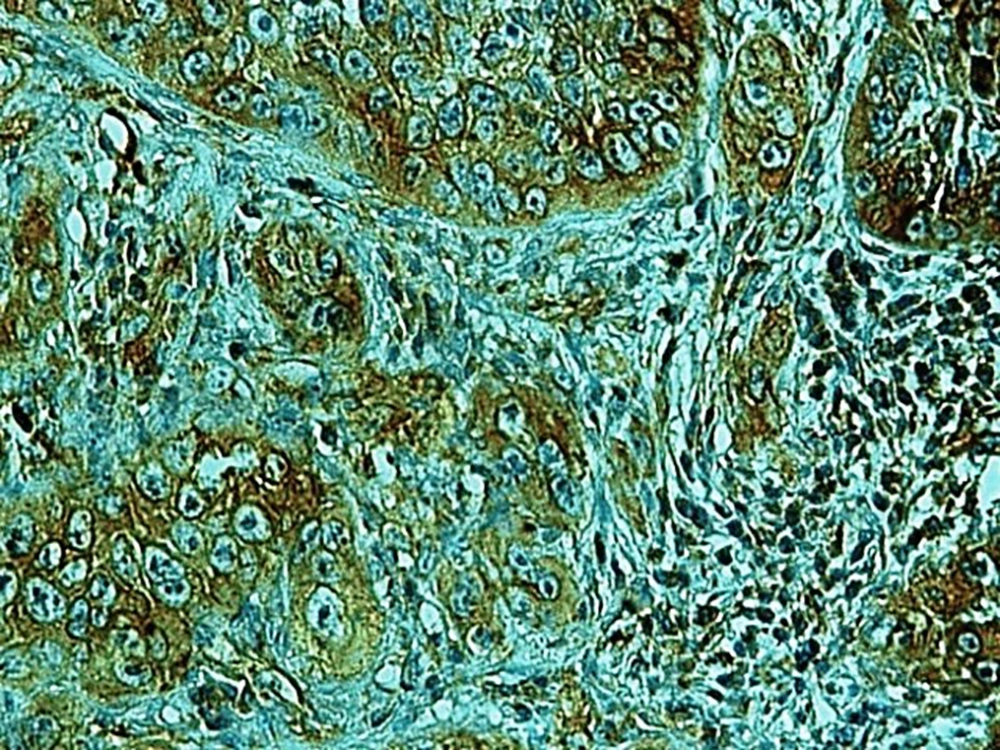
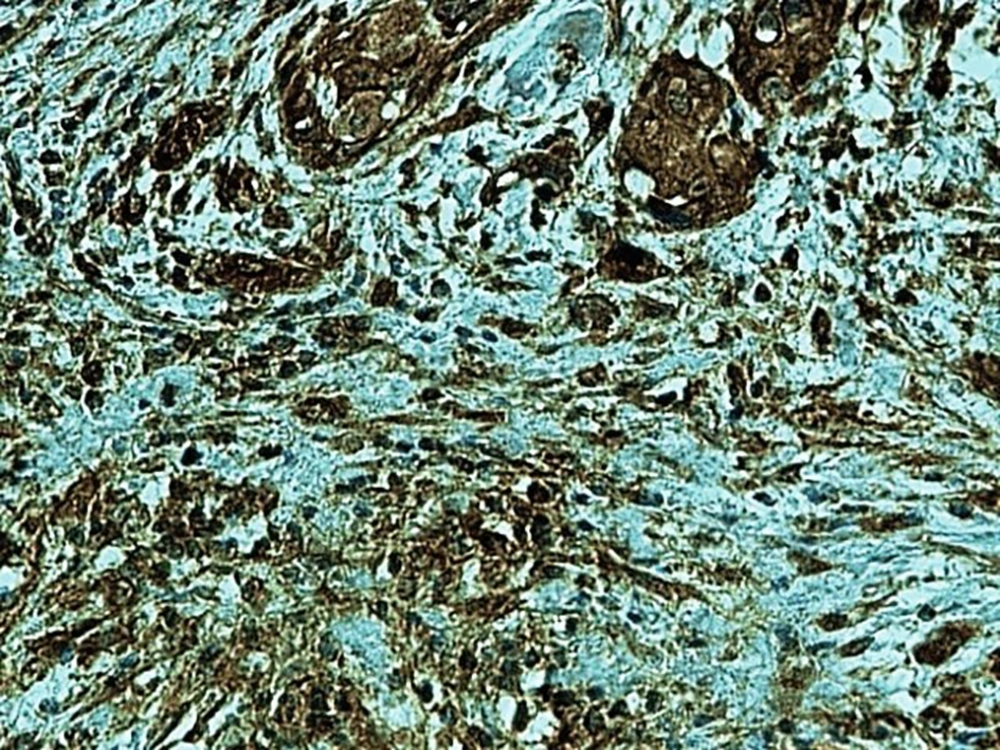
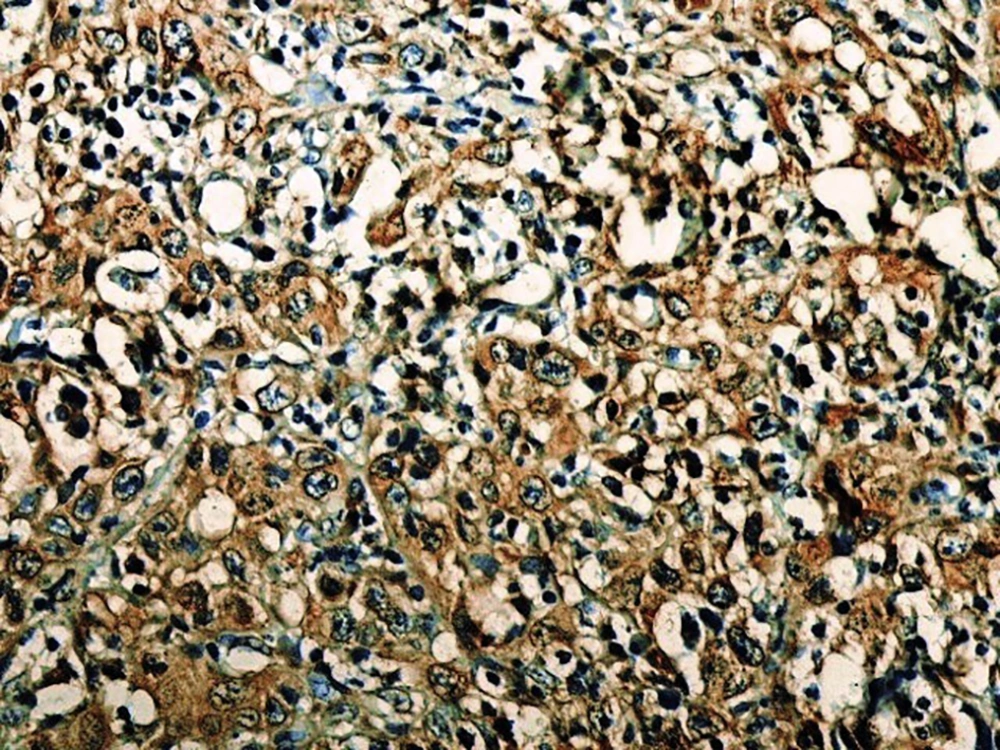
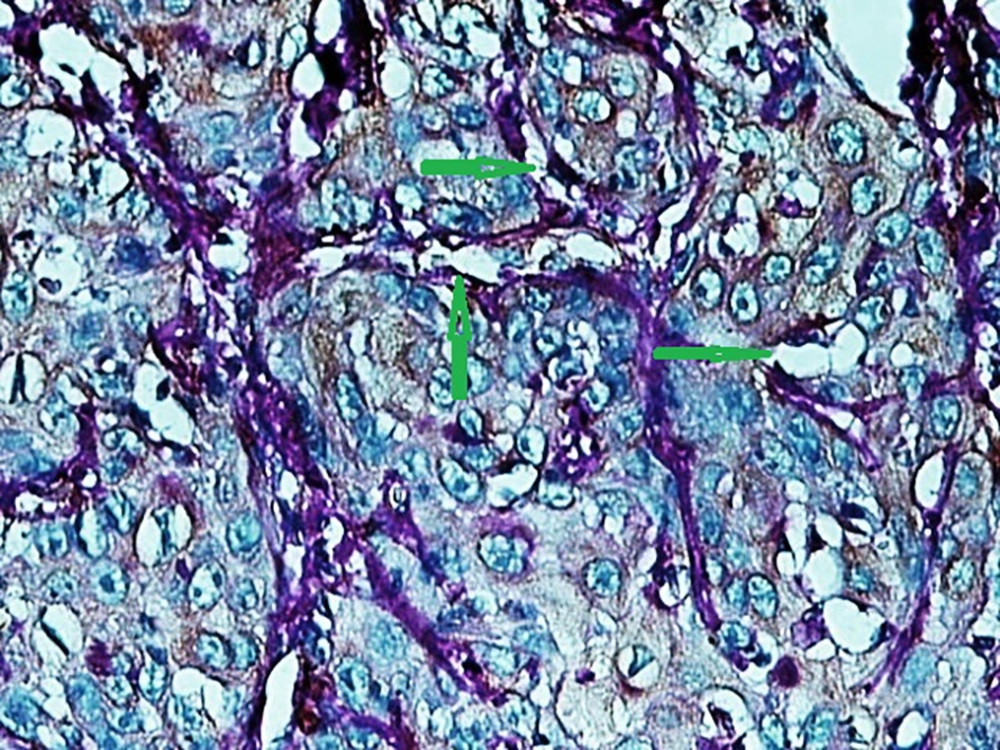
In the present study, as indicated by immunohistochemical staining, the expression of endocan was significantly increased in cancer samples compared to the normal oral tissues. All tissue samples (100%) showed a moderate to the strong expression level of endocan. In addition, there was a significant positive correlation between the expression level of endocan and different grades of cancer tissue samples. A previous study on hepatocellular carcinoma demonstrated a strong endocan expression in the cytoplasm of tumor cells compared to normal tissues (36). In a study on colorectal carcinoma, a strong expression level of endocan was detected in tumor cells (37). According to the published work on meningioma and glioma, endocan expression showed a steady incline from the controls to the most malignant form of the tumor groups. The authors suggested a positive correlation between the endocan expression level and the degree of malignancy (38). These findings may confirm the role of endocan in cancer cell differentiation. Besides, it can be concluded that endocan functions as an oncogene; therefore, it can be considered as a therapeutic target in OSCC. However, a study on gastric cancer indicated a negative correlation between the expression level of endocan and histological differentiation. In this study, of 35 poorly differentiated cases, only 17% of the cases demonstrated strong immunostaining for endocan, but 56% of the well and moderately differentiated cases showed strong immunoreactivity for endocan. The authors suggested that the lower expression of endocan may contribute to the development of gastric cancer. They also suggested that endocan functions as a tumor suppressor gene (18). Additionally, a study on colon cancer revealed that the expression of endocan was correlated to tumor size, depth of invasion, lymph node metastasis, distant metastasis, and staging of the tumor (39). A previously published study on pituitary adenoma suggested that the overexpression of endocan enhances tumor cell aggressiveness (14). It was previously reported that endocan can be used as a biomarker to predict tumor invasion and angiogenesis in pituitary adenoma (40). In invasive bladder cancer, from 46 samples, 50% indicated strong immunostaining of endocan. The authors suggested that endocan expression on tumor blood vessels increases the invasiveness of bladder cancer (41). According to studies, there is a significant correlation between endocan expression and cancer metastasis (12, 42). Based on the immunostaining in the current investigation, strong endocan expression was strongly found at the invasive front and in the vascular endothelial cells mostly close to the periphery of tumor islands. Additionally, endocan-positive tumor cells were detected around the blood vessels mainly in the high-grade tumors (Figures 1 - 3). Invasion and metastasis are the final steps of cancer development. The periphery of the tumor islands (invasive front) and perivascular areas make up the pre-metastatic niche (5, 8). Collectively, it is suggested that elevated endocan expression in tumor cells and vascular endothelial cells may play an indispensable role in tumor development and aggressive growth.
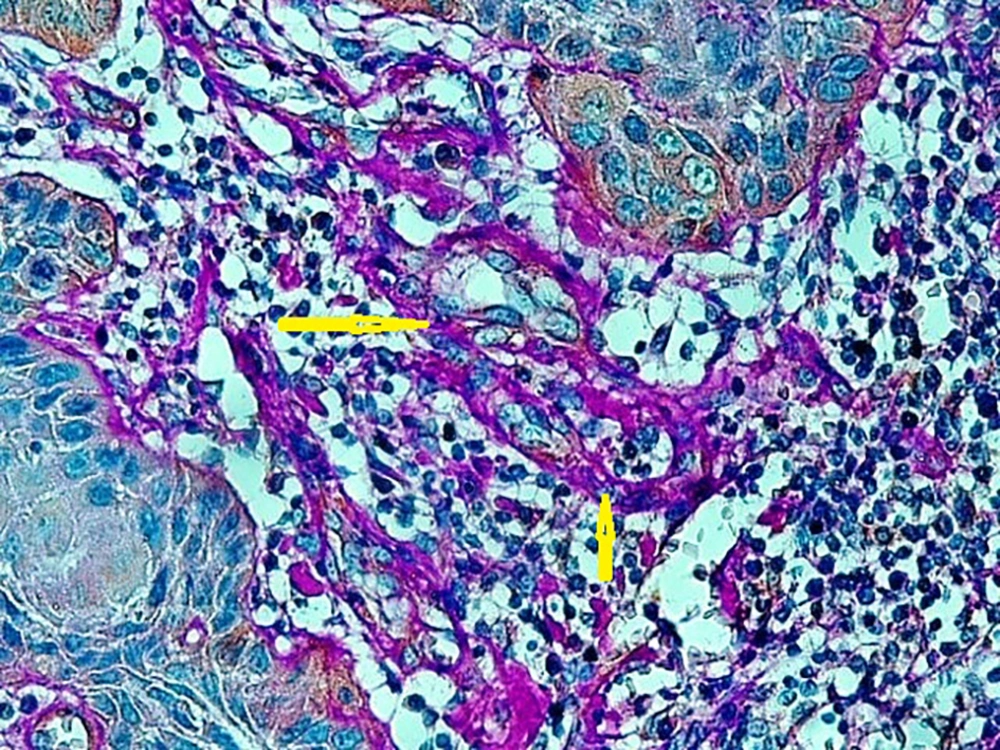
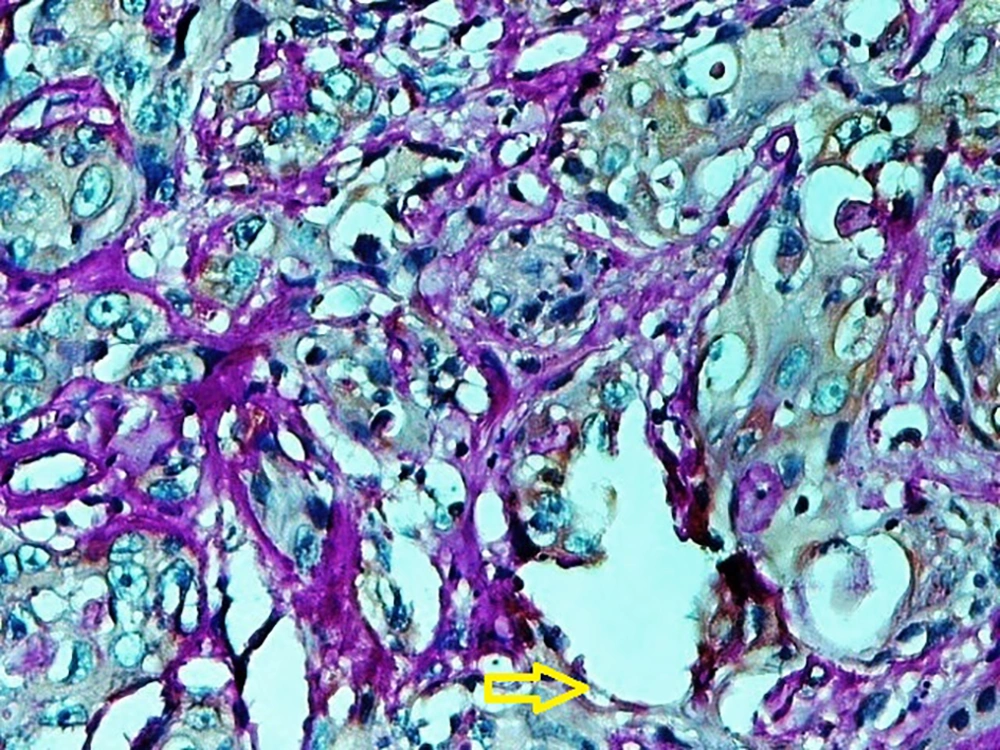
A published study on the expression of endocan in cancers indicated that the strong expression of endocan in high-grade cancers may suggest its contribution to VM formation, (23, 43). VM formation involves tumor growth and cancer metastasis and in OSCC, it is correlated to high-grade tumors. On the other hand, VM formation is correlated to tumor plasticity and CSCs, a small subpopulation of cells within tumors, which are capable of self-renewal, differentiation, and tumorigenicity (8, 26). Supporting documents indicate that in highly aggressive epithelial cancers, VM formation promotes the overexpression of the mesenchymal phenotype through EMT phenomenon, which converts immobile epithelial cells to migratory mesenchymal cells (5, 44). In the current investigation, some detached cells, especially around the vessels expressed endocan, which may demonstrate the acquisition of EMT phenotypes by CSCs. In addition, VM formation lined by endocan-positive cancer cells was found in 27.8%, 66.7%, and 88.9% of low grade, intermediate grade, and high-grade tissue samples, respectively (Figures 4 - 6). Previous investigations have shown the role of CSCs and EMT in VM formation (8, 23). These results may provide enough documents for the role of endocan expression in tumor growth and the risk of metastasis through VM formation, CSCs, and EMT. Besides, these results may suggest endocan as a biomarker for VM formation, CSCs, and EMT phenomenon.
A previous investigation on colon cancer samples indicated the increased expression of endocan in 53.1% of the cases. The authors drew the conclusion that elevated endocan expression occurs under hypoxic conditions. In a published investigation on non-small cell lung cancer, intra-tumoral, and peri-tumoral vascular endothelial cells exhibited a strong expression of endocan in all cases. The authors concluded that the high expression of endocan in non-small cell lung cancer could reflect the response of cancer endothelial cells to pro-angiogenic growth factor stimulation and the endothelial cells are the main source of endocan (12). Endocan expression was rarely found in the endothelial cells of normal tissues such as pituitary gland tissue (13). Besides, a previous study on cultured endothelial cells has demonstrated that endocan promotes the mitogenic and migratory activities of vascular endothelial growth factor (VEGF)-A and -C (45). VEGF, one of the main factors involved in tumor angiogenesis, stimulates endocan secretion by endothelial cells (27). The endocan expression is regulated by some angiogenic factors such as VEGF and also by inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) (16). Endocan has been shown to be activated by VEGF in human umbilical vein endothelial cells. Additionally, the expression of endocan has been indicated in endothelial cells of newly growing tumor vessels (46). In addition to tumor vessels, extracellular matrix (ECM) and stromal cells contribute to tumor growth and cancer progression (8, 28). In the current study, endocan expression was also detected in the stromal endothelial cells. In a study on 150 hepatocellular carcinoma surgical samples, the endocan overexpression was detected in 40% of the samples restricted to the stromal endothelial cells. The authors found that patients with endocan positivity in stromal cells had a higher rate of overall recurrence (47). Endocan positivity was also found in 76% of the gastric cancer tissues. Interestingly, in this study, vascular endothelium of peri-tumor areas showed higher endocan positivity (92.5%) (46).
MVD count is another factor revealing the tumor behavior and endocan-MVD is a valuable factor to predict cancer behavior (46). In a previous study on epithelial ovarian cancer, endocan-MVD was positively correlated to the tumor staging (44). In previous studies on OSCC and mucoepidermoid carcinoma, MVD count was higher in intermediate and high-grade samples compared to that of the low-grade cases (8, 23). In the present study, the expression of endocan was demonstrated in the endothelial cells of tumor center vessels, as well as in the peri-tumor vascular endothelium. Besides, higher MVD count was mainly indicated in intermediate and high-grade samples (Table 1). Hence, it can be noted that the higher MVD count and elevated expression of endocan in tumor vessels are crucial events in cancer formation, tumor differentiation, angiogenesis, and tumor invasion.
Collectively, the current study could be another proof for the previous hypotheses regarding the role of CSC, EMT, VM channel formation, and angiogenesis in oral cancer development and metastasis.
5.1. Conclusions
According to the results of the current study, the increased expression level of endocan was detected in the tumor cells and endothelial cells of OSCC and it was significantly correlated to the tumor cell differentiation. These results suggest that endocan mediates tumor progression possibly via a few mechanisms such as CSCs, EMT, and VM formation. Further studies are needed to reveal the mechanism, by which endocan functions as an oncogene. In addition, endocan could be considered as one of the tumor markers and a possible new target for cancer therapy. The expression level of biomarkers differs between diseased cells and normal cells. Thus, detecting the altered expression level of biomarkers can help in the diagnosis of diseases.