1. Background
Renal cancers account for 2% to 3% of all cancers, while renal clear cell carcinoma (RCC) accounts for 90% of all kidney cancers (1-4). The newly patients diagnosed with renal clear cell carcinoma in the U.S. were 61560 in 2015 and mortality rate is estimated to be around 14080 deaths (5). Patients with RCC suffer from inactivation of von Hippel-Lindau (VHL) gene in 50% to 80% cases (6-8). The product of VHL gene has a crucial role in down-regulating hypoxia-inducible factor (HIF)-1α expression (9, 10). Inactivation of this gene leads to accumulation of HIF-1α, which increases transcription of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (11-13). The increase in HIF-1α is accompanied by tumor invasiveness, metastasis, and poorer survival. The prognosis for patients with RCC is dependent on the stage of disease. In loco regional disease up to 40% have a relapse with metastasis after nephrectomy (14, 15); so, the possibility of micro-metastasis should be considered for these patients, which might present as distant metastasis (16, 17). The 5-year survival rate is 53% for loco regional disease (18). The stage IV of the disease is divided into two groups; in the first group, the tumor develops beyond Georta’s fascia meaning perirenal fat, which can include direct involvement of adrenal gland in surrounding areas or lymph nodes (T4, any N, and M0); the overall survival of patients with RCC (T4, any N, and M0) might reach 40% (19) and the second group includes patients with remote metastasis (any T, any N, M1) (20), the OS is about 8% (19). One of the models to evaluate the risk of relapse is in addition to the model the AJCC TNM classification (version 2010) (20); model is the University of California Los Angles Integrated Staging System (UISS). Based on this, patients in T3b and T3c are at high risk for relapse. Therefore, systematic therapy is recommended to decrease recurrence rate. We started the treatment of target therapy in the patients with loco regional disease in addition to the metastatic group. The standard treatment for RCC has been interleukin combined with interferon for many years (21), but it is associated with minimum therapeutic effect and significant toxicity, though nowadays, targeted therapy is the treatment of choice in the world (22). One of the limitations of targeted therapy is that the medicine does not provide long-term remissions and the body develops drug resistance (13). Thus, sequential targeted therapy or combination therapy is recommended (23). The objective of combination therapy is to increase treatment efficacy and reduce drug resistance (22). Drug toxicity is also important in combination therapy because an increase in toxicity increases patients’ non-compliance and mortality rate. A strategy employed in such circumstances is dose reduction and maintaining the maximum possible dosage until the end of treatment. The increase in toxicity has practically led to failure of targeted therapy; thus, we decided to try combination therapy and combine targeted therapy with immunotherapy. Also, considering limited studies in the past and in order to avoid dose reduction of targeted drugs, which happens in combination therapy, the targeted therapy drug dosage was the standard starting dose; however, a low dose immunotherapy was added. Few studies have shown that reducing sunitinib might reduce drug efficacy, too.
2. Methods
The cohort study was approved by regional Ethical Committee at Kermanshah University of Medical Sciences (Ref. No.: IR.KUMS.REC.1394.301) and 22 patients with loco regional or metastatic aged 18 to 70 years were selected based on inclusion criteria from July 2013 to April 2016 from provinces of Kermanshah, Lorestan, Ilam, and Kurdistan. The sampling method was non-random and easy or available.
Eligibility criteria included a diagnosis of loco regional or metastatic RCC and age between 15 and 75 years. Exclusion criteria included patients with irreversible major organ failure, significant hepatic or renal dysfunction, and a left ventricular ejection fraction less than 40% were ineligible. We used the following formula for the calculation of sample size based on survival rate from previous studies:
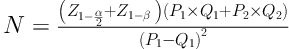
We used Kaplan-Meier estimator to estimate survival function for time to death. SPSS 17.0 for windows was used for all statistical analyses. Since two groups of patients were not the same, there was no comparison between the two groups and we did not need P value. Patients at each stage were compared with prior patients with the same stage. The control groups were historical. The patients’ information is presented in Table 1. The basic medical evaluation for all patients included taking medical history, physical examination, complete blood count, biochemical profile, electrocardiogram, lung and abdomen computed tomography (CT) scan, and bone nuclear scanning. Another inclusion criterion was scoring above 70% in terms of Karnofsky performance status. The criteria for evaluating the response to treatment included CT scan, magnetic resonance imaging, and nuclear scanning after each cycle for the first 4 cycles and, then, every other cycle until the treatment ended. The participants received interferon injection twice a week in 5 million units in combination with 50 mg of sunitinib per day before sleep for 4 weeks, followed by a two-week break (24). The combination therapy was continued for at least 1.5 years meaning minimum 12 cycles in patients with loco regional (T4, N1 - N0, M0), or until the disease progressed or unacceptable side effects appeared or patients with metastasis (any T, any N, M1) were unwilling to continue. If it was necessary to reduce the sunitinib dosage, it would reduce by 50 mg on the first day, 50 mg on the second day, and discontinued on the third day. The primary endpoint of this study was the objective response (reaching full remission and partial remission) and the secondary endpoint was overall survival rate (starting from the time of registration until dying for any reason) and health of combination therapy in terms of toxicological profile. In case of disease progression, the second medicine, sorafenib (Nexavar), in combination with interferon was used this replaced sunitinib if it could stabilize the disease (25).
3. Results
The mean duration of patients’ follow-up was 23 months. The mean age of the patients was 56 years. The performance status (PS) was zero in 18 patients and 1 in 4 patients. The epidemiological investigation into age showed that 4 patients (18.2%) were in the 41 to 50 year age group, 4 patients (18.2%) were younger than 40 years, 7 patients (31.8%) were in 51 to 60 year age group, and 7 patients (31.8%) were in 61 to 70 year age group. There were 12 female patients against 10 male patients. Ten patient were in metastasis (any T, any X, M1) and 12 patients were in loco regional (T4, any N, M0). Among the participants, 2 patients had adrenal involvement, 10 patients had Gerota’s fascia involvement, 10 patients had visceral metastases (including lung, bone and liver), 4 patients had lung metastasis, 2 patients had liver metastasis, 4 patients had extensive abdominal involvement, and 6 patients had bone involvement. Most patients had multiple metastatic sites and while lymph nodes were not evaluated in 11 patients, 6 out of 10 patients, who underwent the evaluation, had lymph node involvement. Eight patients (36.4%) had smoking history, 6 patients (27.3%) had high blood pressure, 2 patients (9.1%) had accompanying diabetes, 2 patients simultaneously had another cancer, 2 patients (9.1%) had accompanying kidney stones, 10 patients had renal vein thrombosis, and 6 patients (27.3%) had inferior vena cava thrombosis. Patients showed different clinical presentations as 7 patients (31.8%) had an abdominal mass, 19 patients (86.4%) had pain, 7 patients (31.5%) had hematuria, 7 patients (31.8%) had pain and abdominal mass, and 2 patients (9.1%) reported chest pain. The side effects of therapy were examined and 4 patients out of 22 suffered from skin rashes, 15 patients (68.2%) suffered from exhaustion, 2 patients developed diarrhea, 2 patients (9.1%) developed stomatitis, 3 patients (13.6%) suffered from thrombosis of lower extremities, 9 patients (40.5%) developed a skin lesion that turned their skins light yellow, and 3 patients (13.6%) had bouts of vomiting. A reduction in platelet count was observed in 40% of patients and reduction in WBC and neutrophil count was observed in 10 patients. The side effects of the therapy were observed during the first 4 weeks of treatment in most patients. The medication toxicity is presented in Table 2. Totally, sunitinib was discontinued in 6 patients. In 4 of these patients, disease progression caused the discontinuation of sunitinib, and 3 of them started receiving sorafenib in combination with interferon. Although the disease was stable for 60 days, it then progressed, and all 3 patients eventually died. The only patients who survived had resumed sunitinib (26) while having interferon in combination with sorafenib (Nexavar), which is still being administered. Ultimately, 3 patients died due to disease progression and one patient died due to heart attack, which was not related to the treatment. Among 22 patients being examined for side effects of the treatment, 12 (54.4%) patients had a short break from taking sunitinib and one patient took a break from receiving interferon. Finally, 12 patients (with T4, N1, N0, M) are in full remission and from 10 patients with visceral metastases, 1 patient is in full remission, 2 patients have stable condition, and 7 (31.8%) patients are in partial remission (Table 3).
| Variables | Valuea |
|---|---|
| Age, y | 56 (30 - 67) |
| Male | 10 (45.5) |
| Female | 12 (54.5) |
| PS 0 | 18 (81.8) |
| PS 1 | 4 (18.2) |
| Nephrectomy | 12 (54.5) |
| Metastasis lung | 4 (18.2) |
| Metastasis liver | 2 (9) |
| Metastasis extensive lymph node | 4 (18.2) |
| Metastasis bone | 6 (27.2) |
a Values are expressed as median (range) or No. (%).
| Adverse Event | Grade 1, 2 | Grade 3, 7 | Total |
|---|---|---|---|
| Fatigue | 15 (67.5) | 1 (4.5) | 16 (72.7) |
| Diarrhea | 2 (9) | 0 | 2 (9) |
| Stomatitis | 2 (9) | 0 | 2 (9) |
| Lemon yellow skin | 9 (40) | 0 | 9 (40) |
| Influenza-like illness | 1 (4.5) | 0 | 1 (4.5) |
| Vomiting | 3 (13.5) | 0 | 3 (13.5) |
| Pyrexia | 4 (20) | 0 | 4 (20) |
| Leukopenia | 9 (40) | 3 (13.5) | 12 (54.5) |
| Thrombocytopenia | 5 (22.5) | 2 (9) | 7 (31.8) |
a Values are expressed as No. (%).
| Variables | Valuea | |
|---|---|---|
| CR | 1 (10) | PD progressive |
| PR | 7 (70) | 2 |
| SD | 2 (20) | 2 |
| PORR | 80 |
Abbreviations: CR, complete response; PD, progressive disease; PORR, primary objective response rate; PR, partial response; SD, stable disease.
a Values are expressed as No. (%) or percentage.
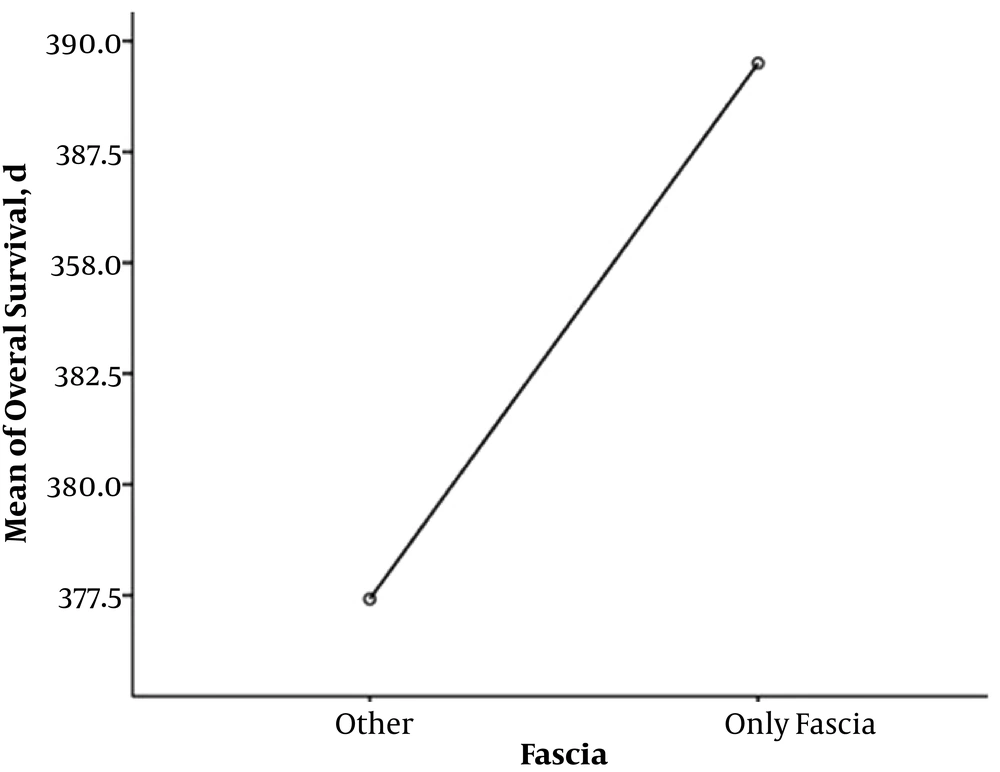
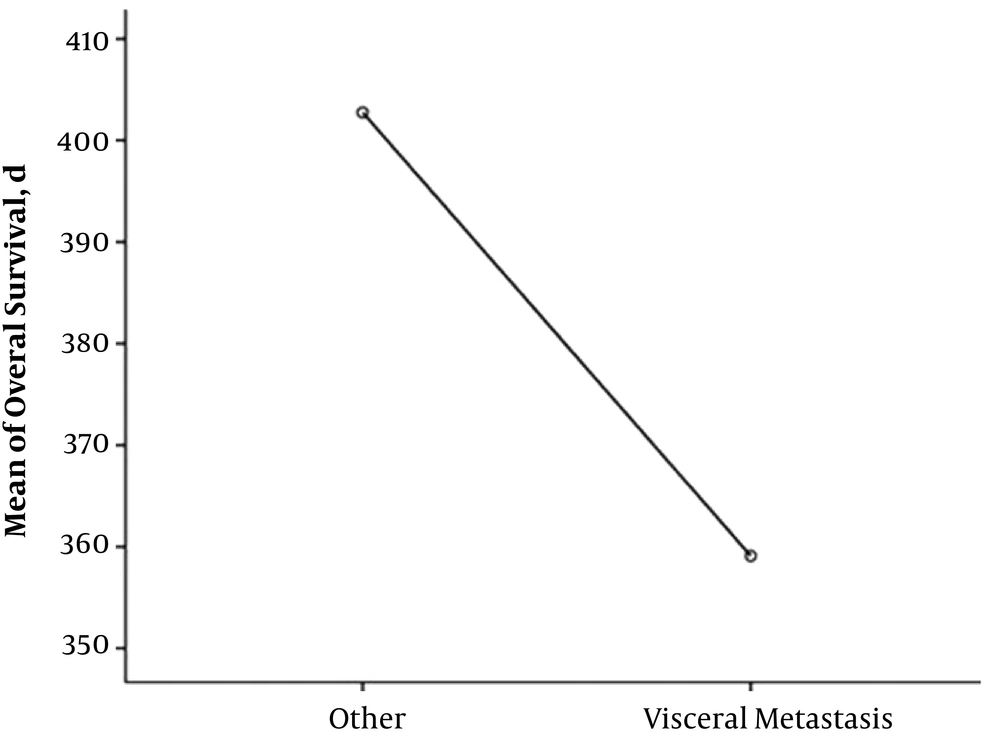
Totally, 18 patients (81.9%) are alive, and 13 patients (59.1%) from the total 22 were in full remission. The mean overall survival of two patients with adrenal involvement was 15.6 months. The mean overall survival in 10 patients with Gerota’s fascia involvement was 12.9 months (minimum four and maximum 20 months) (Figure 1), and the mean overall survival of 10 patients with visceral metastasis was 12 months (Figure 2). The overall survival of total patients was 13 months.
4. Discussion
There are not many studies on the use of target therapy in cases of loco regional disease. The aim of our study was to use target therapy in cases of loco regional use along with its use in metastatic patients. The reason for this decision is the presence of a micro-metastasis phenomenon that can cause recurrence of the disease in loco regional patients. The targeted therapy sunitinib is effective in treating advanced renal carcinoma in two important ways: Affecting vascular endothelial growth factor and mammalian target of rapamycin 1. The response rate to sunitinib in targeted treatment is 30% to 40%, but the disease progressed after a period of 6 to 15 months, which indicates rise of resistance (27). Some of the limitations of using targeted therapy include little chance of reaching a complete remission and rapid progression after discontinuing the drug, and impossibility of saving sunitinib treated patients due to rise of resistance in long-term use, which might also be due to resistance against other genes rather than von Hipple-Lindau (VHL) gene. The mechanism of drug resistance is not fully understood yet, but it is claimed that resistance develops when genetic mutations lead to activation of already inhibited routes or prevent the drug to bond to the target site through changing drug-target interactions. Clinical data show that resistance to targeted therapy is temporary because shifting to another treatment leads to disease regression. Overcoming the resistance would enhance drug efficacy. According to some hypotheses and available information, drug resistance might decrease if interferon is used with targeted therapy, which is why interferon was also used in this study. Interferon has antitumor activity as an immunotherapy and anti-VEGF activity. There is evidence supporting special activity of mammalian target of rapamycin 1 in inducing interferon response, which shows the synergistic effect of sunitinib and interferon against renal cancer cells. Targeted therapy drugs, like suntinitib, result in a high expression of mammalian target of rapamycin. Interferon also results in enhancing natural killer (NK) cell activity and immunization of tumor cells and acts via immunization for destroying malignant cells through cytotoxic CD8+ cells (28). Interferon also induces apoptosis, down-regulates angiogenesis, and promotes cell proliferation through inducing cyclin-dependent kinase inhibitors. Interferon-α increases p53 expression (29) as a tumor suppressor, which has an important role in controlling angiogenesis and apoptosis and intensifies deadly responses to genotoxic stressors. The study conducted by Motzer et al. (30) on combination therapy of interferon with suntinitib reported dose reduction in 18 patients out of 25 so that the suntinitib dosage was reduced to 37.5 mg and 3 million units of interferon was administered subcutaneously 3 times a week; however, even such a low dosage was not tolerable in long term. In Motzer et al.’s study, 100% of patients suffered from fatigue and 76% and 56% suffered from diarrhea and vomiting, respectively. So, considering the side effects of the treatment in that study, we decided to reduce the dosage of combination therapy, but it was only the interferon dosage that was reduced and suntinitib dose reduction was avoided. If it was necessary to reduce the dosage or discontinue the medication, the break was short and the suntinitib dose reduction was little since there was evidence, indicating that reducing the suntinitib dose would reduce drug efficacy, too. Comparing the side effects of the present study with other studies that employed combination therapy (53%) or used suntinitib alone (44%) or used interferon alone (33%), our research results did not indicate more severe and threatening side effects; thus, we would recommend our treatment as it was less toxic and safer. The most common side effects observed among our patients were fatigue, weakness, lethargy and facial skin color changes to light yellow. The percentage of patients with side effects of grade 3 to 4 thrombocytopenia and leucopenia was as low as 20%. Also, the dose of suntinitib was changed in only 12 patients and toxicity did not result in discontinuing suntinitib. In Muntzer’s research that studied 146 patients, the drug dosage was reduced for 36.3% of patients and it was discontinued in 15.8% cases, while the reason for discontinuation of the medication in our study was disease progression. Our strategy for active response toward toxicity associated with treatment included resuming treatment with suntinitib as soon as possible. The strategy should include educating and monitoring patients, and resuming the treatment as soon as the signs of improvement from drug toxicity appear, and dosage should not be reduced and treatment should not be delayed or discontinued. None of our patients suffered from decreased cardiac output. This combination therapy of sunitinib with interferon had a significant response rate so that the overall response rate was 80%. The greatest limitation of the present study was small number of participants. In a recent study by Ravaud et al. (31), sunitinib as adjuvant treatment significantly improved DFS versus placebo in patients at high risk of recurrence of RCC following surgery (S-TRAC).
4.1. Conclusions
Considering the low survival rate of patients with loco regional stage and metastatic disease, one of the aims of the study was to examine the survival rate when using sunitinib therapy in loco regional stage. Another aim of the study was to investigate the effect of combined therapy on survival rates in patients with metastatic disease. Compared to prior patients with same stage (historical control), increased survival rates were significant in both groups of patients, especially in the loco regional group.
The most important finding of this research is the fact that a combination therapy of immunotherapy with targeted therapy might be effective in patients with loco regional renal-cell carcinoma at high risk for tumor recurrence after nephrectomy and metastatic renal carcinoma.