1. Background
Nowadays, the prevalence of cervical cancer and its death rate are still high among women around the world and has the forth rank in incidence as well as mortality (1, 2). Although there are many strategies for declining its symptoms such as surgery, radiotherapy, chemotherapy, and their combination following tumor resection and recurrence is usually occurring due to cancer cell resistance to therapy (3). Also, damaged normal cells are another drawback of these therapies. Hence, today many studies have been accomplished to investigate the anti-cancer characteristics of natural agents such as medicinal plants (4-6).
Crocin (C44H70O28) with molecular weight of 976.96 g/mol is one of the main water-solving carotenoids of saffron stigma (7). Several investigations illustrated that crocin could be used for curing many diseases such as cancers (8), neurological disorders (9), cognitive complications (10), immune system diseases (11-13), hepatocyte damages (14), and many kinds of inflammation (15). In the last decades, many researchers investigated the anti-proliferative properties of crocin on different cancers through different pathways, such as apoptosis (7). Sun et al. stated in their survey that crocin could prohibit the growth and tumorigenicity of HL-60 cancer cells (16). Before them, in 1996 Escribano et al. compared the invasive effects of 4 compounds of saffron on Hela cells, too; crocin was the most effective one (17). Aung et al. also demonstrated that crocin had anti-proliferative effects on some cancer colorectal cell lines (18). In previous studies, anti-growth and pro-cell death effects of crocin have been reported In-vivo (19) and In-vitro (20). Likewise, changes in the Bax/Bcl-2 ratio, activation of caspases, and cell cycle arrest by an alteration in cyclin D1 and p21 mRNA levels were studied (21). Crocin can be effective in cell cycle arrest at G0/G1 phase via changing the expression of some key proteins in different cancers such as Bax and Bcl2 (22). However, its anti-cancer molecular mechanism is not yet completely known. So, crocin notably has a chemo-preventive activity because of its low toxicity and potent efficacy in cancer (23).
2. Objectives
We aimed at assessing the inhibitory effect of this carotenoid in combination with epirubicin as one of the main chemotherapy drugs, on the proliferation of cervical cancer cells for first time.
3. Methods
3.1. Crocin Preparation
According to our recent studies, Crocin was isolated and purified from Iranian saffron (21).
3.2. Cell Cultures
The OV2008 cells were kindly prepared from Prof. Tsang (University of Ottawa, Canada) as chemo-sensitive cervical cancer cells. They were cultured in RPMI1640 cell media supplemented with 10% inactivated FBS, 100 units/mL penicillin, and 100 mg/mL streptomycin (Gibco BRL, Grand Island, NY, USA). After that, the cells were treated with crocin in different concentration (0 - 4000 µm/mL) and epirubicin (0 - 6.25 µm/mL) combination (R = 3) and incubated at 37°C including the atmosphere of 5% CO2.
3.3. Cell Viability Assay
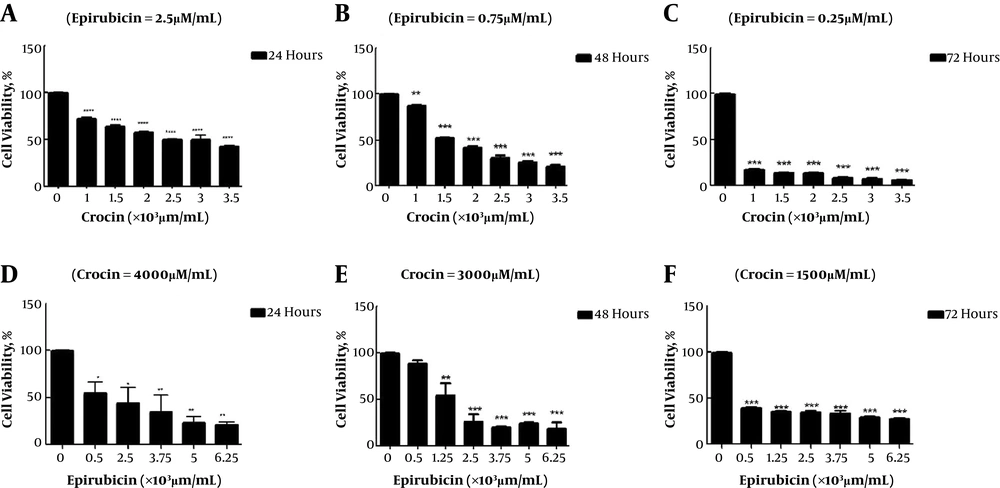
Crocin cytotoxicity effects on OV2008 were measured by MTT assay (Sigma-Aldrich; M2128-1G, USA) according to our former study (3). The absorbance was read at 540 nm by ELISA plate reader (BioTek, Epoch, USA). Furthermore, the IC50 (initial concentration of component that decreased the percentages of live cells to 50%) of these combinations was determined to analyze the cytotoxic efficiency of them by using the dose- and time-dependent curves.
3.4. Appraisement of Apoptosis with Hoechst Staining
After exposing the cells with Crocin and Epirubicin, they were harvested in 6 wells plates and stained by Hoechst 33258 (12.5 ng/mL) to study the percentages of apoptotic nuclear morphology (nuclear shrinkage, condensation, and fragmentation) (20).
To measure synergic effect of crocin and epirubicin, the synergic index (q) was calculated with below formula according to our previous studies (3, 24):
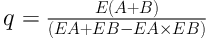
E (A + B) showed the growth suppression rate of crocin and epirubicin. EA and EB were their suppression rates alone, respectively.
0.85 < q < 1.15: Simple addictive effects
1.15 < q < 2: A degree of synergism
q > 2: Significant synergistic effect
3.5. RNA Extraction and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
RNX Plus reagent (Cinnagen, Iran) was used to extract RNA, entirely. 260/280 and 260/230 absorbance ratio were achieved, using nanodrop (BioTek, Epoch, USA). The primer sequences are as follow: β-actin (forward: TGGCACCCAGCACAATGAA, reverse: CTAAGTCATAGTCCGCCTAGAAGCA), Bax (forward: GGTTGTCGCCCTTTTCTA, reverse: CGGAGGAAGTCCAATGTC), Bcl2 (forward: GATGTGATGCCTCTGCGAAG, reverse: CATGCTGATGTCTCTGGAATCT), P21 (forward: GGCAGACCAGCATGACAGATT, reverse: GCGGATTAGGGCTTCCTCTT). The cDNA was synthesized by PrimeScript RT™ reagent kit (Thermo Scientific, USA). The quantities RT-PCR was done by the SYBRGreen master mix (Parstoos, Iran) and ABI Step One TM Real-Time PCR System (Applied Biosystems, Foster City, CA) (3, 24).
3.6. Propidium Iodide (PI) Staining Assay
PI staining kit (IQ product, Netherlands) was used for apoptosis detection following the manufacturer’s instruction. Before that, we treated cells by 3000 µm/mL crocin and 0.75 µm/mL epirubicin. Then, the cells were fixed until the PI staining assay was performed. Next, the percentage of cells undergoing apoptosis and the cells were entered in different cell cycle phases was determined by BD FACSCalibur flow cytometer (Becton Dickinson, Canada) and analyzed by FlowJ software.
3.7. Statistical Analyses
All the experiments were carried out in triplicate, independently, and the results were declared as the mean ± SD, after analyzing by Prism software. For mean comparison of each group to control, the Student’s t test was used (P < 0.05).
4. Results
4.1. IC50 Value
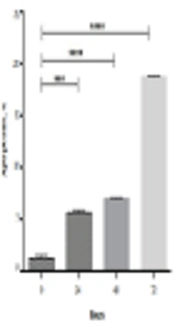
Figure 1 illustrates that crocin and epirubicin prohibit development of the OV2008 cells in a dose- and time-dependent manner at 24 hours, 48 hours, and 72 hours. The graphs illustrated that the cell viability declined by increasing hours and concentration, significantly (P < 0.05 and P < 0.0001). As we shown in Table 1, by increasing time, the IC50 of crocin and epirubicin decreased. Also, the IC50 of crocin and epirubicin combination reduced after 48 and 72 hours. According to the previously mentioned formula, the synergic index (q) was calculated for each time and the results were 0.86, 0.57, and 0.76 for 72, 48, and 24 hours, respectively. As a consequence, after 72 hours, these two components showed a simple additive effect.
| Treatment, µm/mL | 24 h | 48 h | 72 h |
|---|---|---|---|
| Crocin | 5000 | 3000 | 1500 |
| Epirubicin | 2.5 | 0.75 | 0.25 |
| Crocin (constant) + epirubicin (variable) | 0.5 | 1.25 | 0.5 |
| Crocin (variable) + epirubicin (constant) | 2500 | 1500 | 1000 |
IC50 Values (µm/mL) of Crocin and Epirubicin and Their Combination on OV2008 Cell Line
4.2. Apoptosis
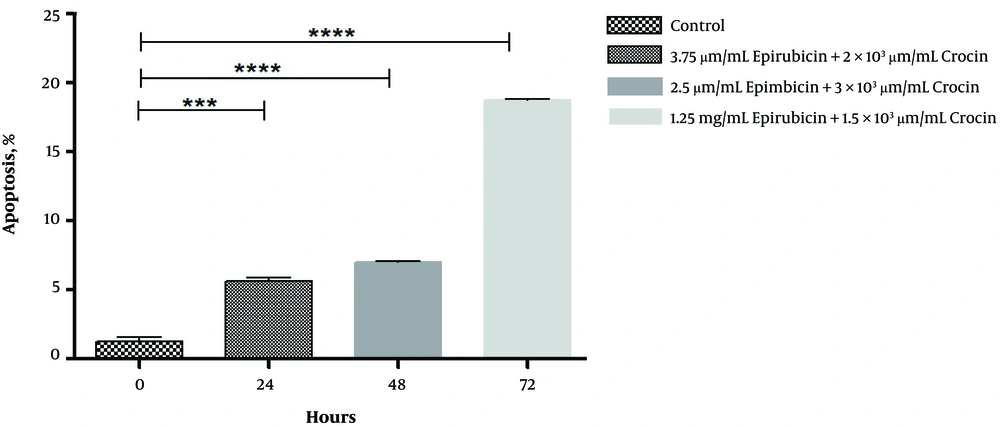
As it was revealed in Figure 2, treated cells with the lower level of epirubicin and crocin after 72 hours may lead to the most apoptosis compared to control (17%). Similarly, in comparison with the control group, the percentages of apoptotic nuclear cells were increased significantly, but around 5% after 24 and 48 hours (P = 0.0001, P < 0.0001).
4.3. Gene Expression
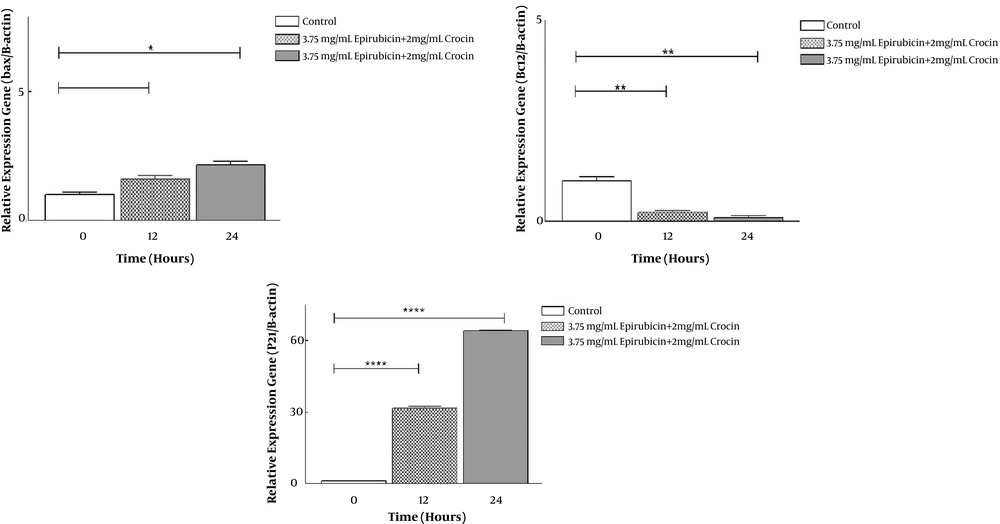
As shown in Figure 3, the selected dose of crocin and epirubicin combination diminished Bcl2 expression level in OV2008 (P < 0.05). These combined components also led to enhancing P21 and Bax genes expression significantly after 24 hours in comparison with the control group, which is more obvious in P21 (P < 0.05).
4.4. Cell Cycle and Apoptosis
Table 2 enounced that after 24, 48, and 72 hours, treated cells by 3000 µm/mL crocin and 0.75 µm/mL epirubicin led to escalating dead cells percentages (subG1) in comparison with control group. Moreover, this combination affected introduced cells in each phase of the cell cycle. So, by inducing cell death, the cells which enter in the cell cycle (phase G1) and the percent of cells in the DNA replication stage (phase S) declined. Thereby, cell proliferation was suppressed after treatment.
| Hours | Phases | |||||||
|---|---|---|---|---|---|---|---|---|
| SUB-G1 | G0/G1 | S | G2/M | |||||
| Control | Treatment | Control | Treatment | Control | Treatment | Control | Treatment | |
| 24 | 7.78 | 35.9 | 66.1 | 24.3 | 49.7 | 26.7 | 18.5 | 22.4 |
| 48 | 3.47 | 18.1 | 71.5 | 40.1 | 48 | 38 | 16.9 | 34.6 |
| 72 | 13 | 25 | 64.3 | 35.9 | 43.5 | 39 | 12.3 | 22.4 |
The Amount of OV2008 Cells in 24 Hours, 48 Hours and 72 Hours Introducing in Different Phases of Cell Cycle Measured by Flowcytometrya
5. Discussion
Cervical cancer is one of the most prevalence neoplasms between women (25). In spite of the progression in therapeutic and diagnostic options, tumor cells resistance to drugs leads to recurrence of cancer (25). In order to overwhelm these complications, scholars tend to catch sight of some innovated therapy by investigating possible antitumor agent in botanies (26, 27).
In the Middle East and some European countries, saffron (Crocus sativus L.) was widely used as a condiment and a herb (28). Obviously, Crocin, as a main component of saffron, has many prominent properties such as apoptotic and anti-cancer effects, which have been demonstrated in several cancers (21, 29-32).
Apoptosis is a highly complex mechanism, which participates in chemo-resistance and done via two particular pathways: The death receptor and mitochondrial pathway. Today, they find out these pathways have interconnected with each other through some molecules (21, 33). In our survey, we demonstrated that Hoechst staining outcomes is in comprise of MTT assay and showed crocin and epirubicin combination can kill OV2008 cells. Furthermore, pursuant to our previous research (3), this aforesaid combination induced survival reduction in OV2008 cell line after 48 hours (crocin constant and epirubicin variable: IC50 = 1.25 µm/mL, crocin variable and epirubicin constant IC50 = 1500 µm/mL) and 72 hours (crocin constant and epirubicin variable: IC50 = 0.5 µm/mL, crocin variable and epirubicin constant IC50 = 1000 µm/mL).We realized that the combination of crocin and epirubicin only in 72 hours had a simple additive effect. To assay their impacts on cell cycle phases, we used flowcytometry technique. The results of the cell cycle analysis showed that this combination increased the percentage of dead cells (subG1 phase) compared to the control group and also affected the percentage of cells, which introduced in each of the cell cycle phases. We also observed that treated cells by 3000 µm/mL crocin and 0.75 µm/mL epirubicin led to increasing the percentages of secondary apoptotic and necrotic cells in comparison with control group. To further illuminate the anti-cancer mechanism of crocin on the OV2008 cell line, we encountered changes of apoptosis-related Bax, Bcl2, and P21 genes by real-time PCR technique. According to our consequences, the combination of epirubicin and crocin decreased bcl2 and increased p21 and Bax expressions over time, significantly as well as our results when we use crocin alone (3). Among many tumor suppressors, P53 plays a critical character in preventing improper cell increase and inducing cell cycle detention in the G1/G2 and S phases. P21 has been identified as a downstream target and primary intermediary of P53, follow up DNA damages (34). It also plays a key role in apoptosis and responds to stress factors by up-regulating p53 protein in order to detain cell cycle. Afterward, it caused barricading transcription of bcl2 and up-regulating Bax gene expression (35). Between Bax family proteins, Bcl2 and Bax act as important agents in the mitochondrial pathway and their ratio immovably leads to drug resistance and apoptosis capacity modulation (33, 36).
Similar studies have already been conducted. These surveys insisted that Crocin exhibited an effective inhibitory effect on proliferation of different cancer cells. For the first time, Tarantilis et al. demonstrated that crocin had an inhibitory effect on HL60 cell line growth (37). In another study, crocin and ethanolic extract of Crocus sativus exhibited a dose-dependent inhibitory effect on skin mice papillomas (38). Aung et al. also investigated the effect of crocin on human colorectal cancer cell lines (HCT-116, SW-480, HT-29) and reported a reduction in cell growth and proliferation (18). Hoshyar et al. accounted that crocin causes apoptosis by increasing bax/bcl2 ratio in AGS cell lines (human gastric adenocarcinoma cells (21). Nowadays, many studies indicated that the combination of crocin with different kinds of chemotherapy drugs may lead to enhance the efficiency of common cancer treatments through declining essential dose of these toxic medicines (32, 39-41). Meanwhile, because crocin is a safe water-soluble carotenoid, it can be used in companion with the little essential dose of chemotropic drugs such as epirubicin to get better results in cancer therapy. Nonetheless, more investigations are needed to enlighten their synergic or antagonist effects in cervical cancerous animal model.
5.1. Conclusions
Overall, the present study demonstrated the more effectiveness and stronger pro-apoptotic effect of crocin and epirubicin on OV2008 cells compare them alone through changing the expression of Bax, Bcl2, and P21 genes.